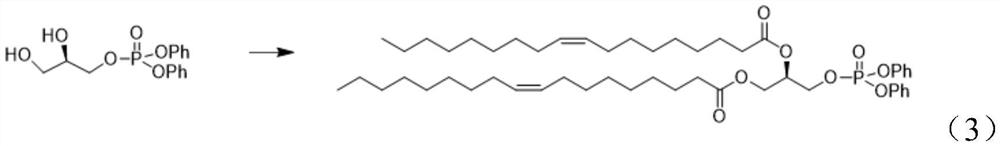
Preparation method of 1, 2-dioleoyl-SN-glycerol-3-phosphorylethanolamine
A technology of phosphorylethanolamine and glycerol, applied in 1 field, can solve the problems of liposome stability, poor membrane fusion transfection efficiency, etc., and achieve the effects of few steps, short routes, and easily controllable conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of DOPE according to the embodiment of the present invention comprises the following steps:
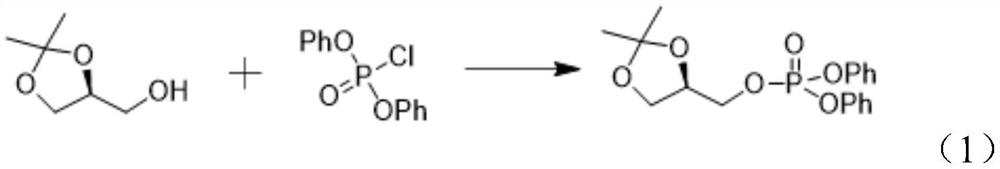
[0028] Step S1, making (S)-glycerol acetal and diphenyl chlorophosphate undergo a nucleophilic substitution reaction under the action of the first base to obtain [(R)-2,2-dimethyl-1,3-dioxolane Cyclo-4-yl]methyl diphenyl phosphate.
[0029] Specifically, the reaction formula is shown in the following formula (1):
[0030]
[0031] Further, in the step S1, the first base is one or more selected from pyridine, triethylamine, DIEA, sodium carbonate, and sodium hydroxide. Among them, pyridine is preferred, and the reaction yield will be higher.
[0032] Further, in the step S1, the molar ratio of the (S)-glycerol acetal, diphenyl chlorophosphate and the first base is 1:(1-1.5):(1-1.8); the reaction temperature is 0~35℃, the reaction time is 1~10 hours. Preferably, the molar ratio of (S)-glycerol acetal, diphenyl chlorophosphate and base is 1:1.0:1.3. ...
Embodiment 1
[0060] (1) Preparation of [(R)-2,2-dimethyl-1,3-dioxolan-4-yl]methyl diphenyl phosphate (I)
[0061] Dissolve 20g (S)-glycerol acetal in 300mL of dichloromethane in a three-necked flask, add 15.6g of pyridine, drop it into an ice-water bath, dropwise add 100mL of diphenyl chlorophosphate in dichloromethane solution, and keep the internal temperature for the reaction 2h. TLC monitored the complete reaction of the raw materials, the reaction solution was washed with 150 mL of 1M HCl, and then washed twice with 150 mL of water, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain [(R)-2,2-dimethyl-1,3- Dioxolane-4-yl]methyl diphenyl phosphate (I) 50.7 g, yield 92%.
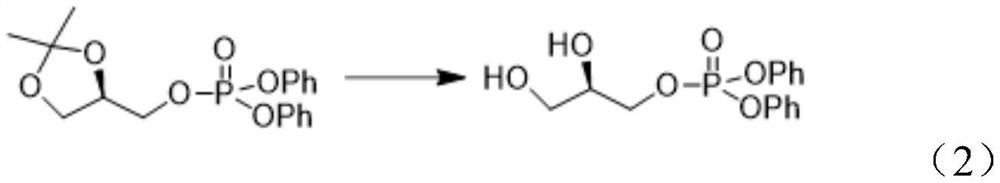
[0062] (2) Preparation of (R)-2,3-dihydroxypropyl diphenyl phosphate (II)
[0063] In a three-necked flask, 20 g of [(R)-2,2-dimethyl-1,3-dioxolan-4-yl]methyl diphenyl phosphate (I) was dissolved in 160 mL of a mixed solvent of methanol and water Then, 1.6 g of p-toluenesulfonic a...
Embodiment 2
[0072] (1) Preparation of [(R)-2,2-dimethyl-1,3-dioxolan-4-yl]methyl diphenyl phosphate (I)
[0073] Dissolve 20g (S)-glycerol acetal in 300mL of dichloromethane in a three-necked flask, add 19.9g of triethylamine, drop it into an ice-water bath, dropwise add 100mL of diphenyl chlorophosphate in dichloromethane solution, keep the inside Warm reaction for 2h. TLC monitored the complete reaction of the raw materials, the reaction solution was washed with 150 mL of 1M HCl, and then washed twice with 150 mL of water, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain [(R)-2,2-dimethyl-1,3- Dioxolane-4-yl]methyl diphenyl phosphate (I) 47.4 g, the yield was 86%.
[0074] (2) Preparation of (R)-2,3-dihydroxypropyl diphenyl phosphate (II)
[0075] In a three-necked flask, dissolve 20 g of [(R)-2,2-dimethyl-1,3-dioxolan-4-yl]methyl diphenyl phosphate (I) in 160 mL of dioxane and water 1.6 g of p-toluenesulfonic acid monohydrate was then added to t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com