Method for efficiently and continuously producing potassium peroxymonosulfate composite salt
A kind of potassium hydrogen persulfate compound salt, high-efficiency technology, applied in the direction of chemical instruments and methods, alkali metal sulfite/sulfite, alkali metal compounds, etc., can solve the difficulty of controlling the precise addition of raw materials, low cooling efficiency, Product quality fluctuations and other issues, to achieve the effect of automatic precision control, small equipment footprint, and less online raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
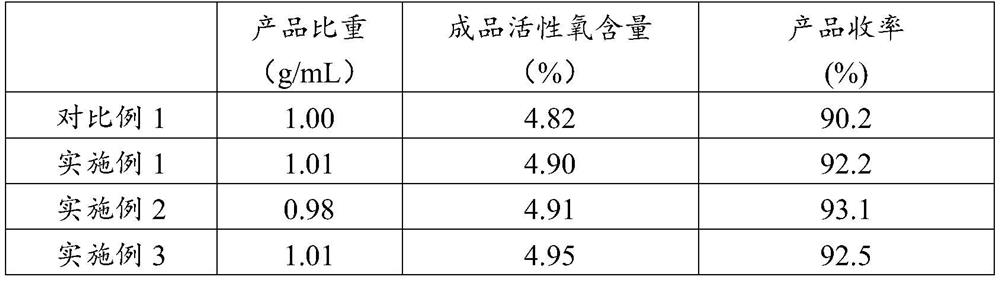
Embodiment 1
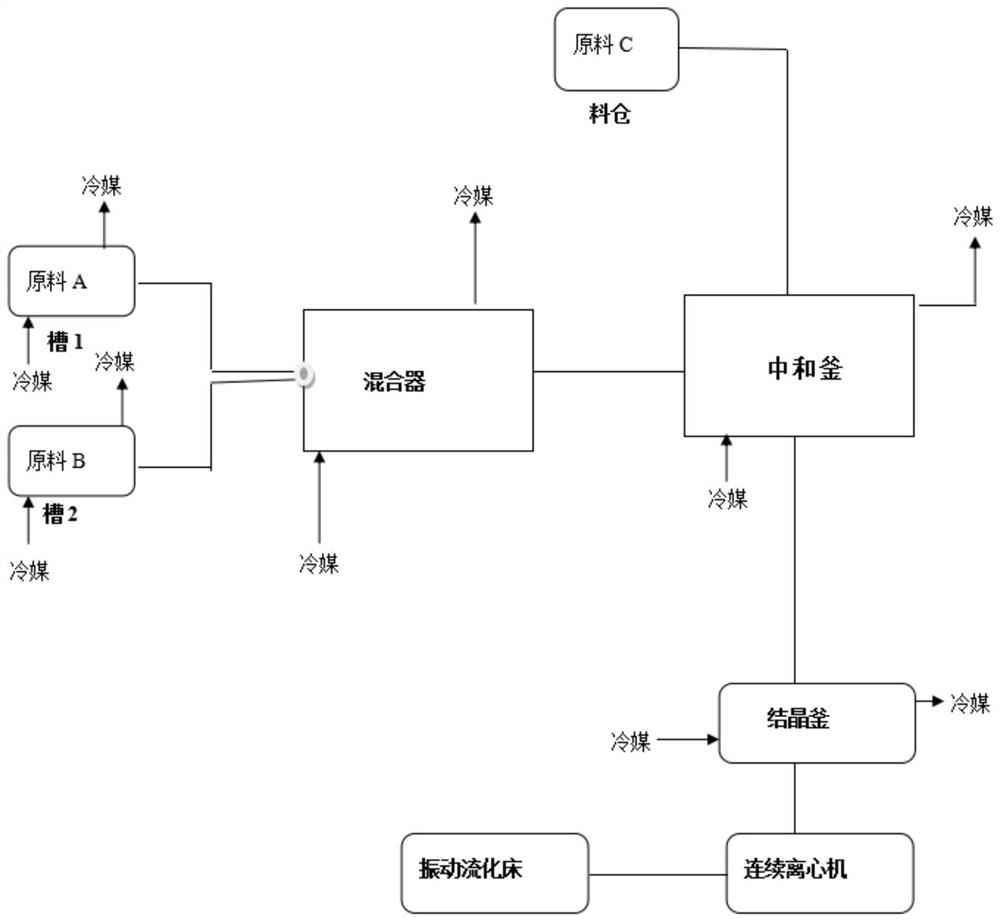
[0028] (1) raw material: weigh 150kg of oxidizing liquid by mass flowmeter, pump it into raw material tank 1, open frozen brine, reduce the temperature of oxidizing liquid to -10 ℃, use as raw material A for standby; weigh 250kg of mother liquor by mass flowmeter, Pump into the raw material tank 2, open the frozen brine, reduce the temperature of the mother liquor to -10 ° C, and use it as the raw material B for standby; weigh 90 kg of potassium carbonate and put it into the silo for standby.
[0029] Reactor equipment: the mixer is a spiral plate mixer with a heat exchange area of 5m 2 .
[0030] Neutralization kettle: The transfer kettle is a 300L stainless steel reactor.
[0031] (2) The raw material A and the raw material B are pumped and pumped into the mixer through the flow meter, and the flow rate of the raw material A is controlled to be 75kg / h, and the flow rate of the raw material B is 125kg / h; the inlet frozen brine is -20 ℃, and the outlet frozen brine is 5 ℃;...
Embodiment 2
[0036] The difference from Example 1 is that the selected alkali metal salt of potassium is KOH.
[0037] (1) Raw material: weigh 150kg of oxidizing liquid by mass flowmeter, pump into raw material tank 1, turn on frozen brine, reduce the temperature of peroxide liquid to -10°C, and use as raw material A for standby; weigh 250kg of mother liquor by mass flowmeter , pumped into raw material tank 2, opened frozen brine, lowered the mother liquor temperature to -10 ℃, used as raw material B for standby; weighed 74 kg of potassium hydroxide and put it into the silo for standby.
[0038] Reactor equipment: the mixer is a spiral plate mixer with a heat exchange area of 5m 2 .
[0039] Neutralization kettle: The transfer kettle is a 300L stainless steel reactor.
[0040] (2) The raw material A and the raw material B are pumped and pumped into the mixer through the flow meter, and the flow rate of the raw material A is controlled to be 75kg / h, and the flow rate of the raw material...
Embodiment 3
[0045] The difference from Example 1 is that the heat exchange area of the mixer is different.
[0046](1) raw material: weigh 150kg of oxidizing liquid by mass flow meter, pump into raw material tank 1, open frozen brine, reduce the temperature of oxidizing liquid to -10 ℃, use as raw material A for standby; weigh 250kg of mother liquor by mass flow meter , pumped into the raw material tank 2, opened the frozen brine, lowered the temperature of the mother liquor to -10 ℃, used as the raw material B for standby; weighed 90 kg of potassium carbonate and put it into the silo for standby.
[0047] Reactor equipment: the mixer is a spiral plate mixer with a heat exchange area of 10m 2 .
[0048] Neutralization kettle: The transfer kettle is a 300L stainless steel reactor.
[0049] (2) The raw material A and the raw material B are pumped and pumped into the mixer through a flow meter, and the flow rate of the raw material A is controlled to be 75kg / h, and the flow rate of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com