Lactobacillus johnsonii and application thereof in degradation of deoxynivalenol and inhibition of pathogenic bacteria
A technology for deoxynivalenol and Lactobacillus johnsonii, which is applied in the field of microorganisms and achieves the effects of being safe, reliable, easy to use and effective.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 - Isolation, screening and identification of Lactobacillus johnsonii NJD412
[0047] The present embodiment adopts the following method to obtain Lactobacillus johnsonii NJD412:
[0048] (1) Isolation and screening of lactic acid bacteria
[0049] A total of 10 different dog feces samples were collected in Xuanwu District, Nanjing, and 0.1 g of feces were weighed and diluted to 10 with sterile normal saline. -4 times, spread on MRS plate (purchased from Qingdao Haibo Biological Co., Ltd., HB0384), cultured at 37°C for 48h, and the plate colonies were preliminarily observed. Aseptically pick a suitable single colony and inoculate it in MRS liquid medium (purchased from Qingdao Haibo Biological Co., Ltd., HB0384-1), and after standing at 37°C for 48 hours, take 10 μL of bacterial liquid MRS plate for streaking. This step was repeated three times to obtain a purified culture strain.
[0050] The DNA of the purified strain was extracted from the purified strain...
Embodiment 2
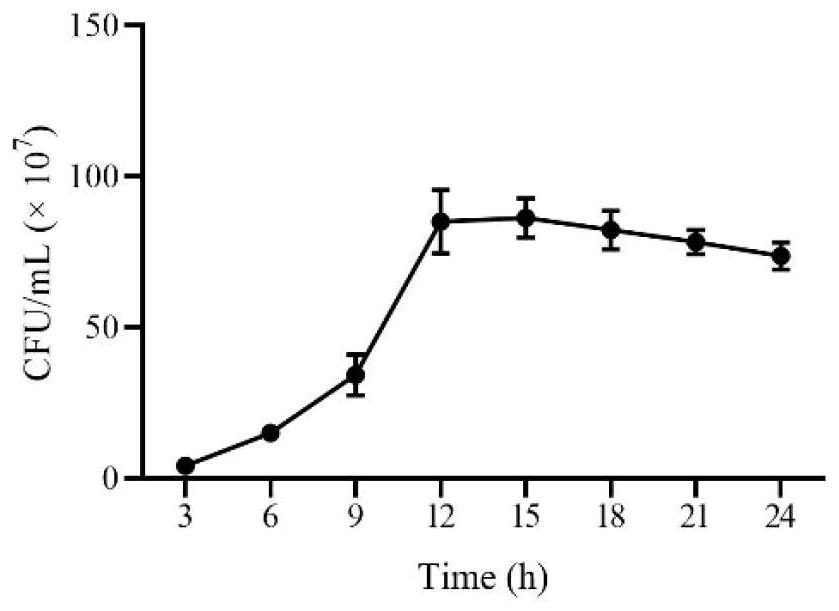
[0061] Example 2 - Growth curve, in vitro acid production, acid resistance and bile salt resistance test of Lactobacillus johnsonii NJD412
[0062] (1) Determination of growth curve
[0063]Lactobacillus johnsonii NJD412 with deoxynivalenol enol degradation ability in Example 1 was activated, and the activated bacterial solution was passaged into 100 mL MRS liquid medium at 5%, placed in a 37°C constant temperature incubator, and allowed to stand. Incubate for 24h, draw 1mL of bacterial liquid every 3h for continuous 10-fold dilution, and adjust the concentration to 10 -1 to 10 -7 , respectively draw 100 μL of the dilution solution and spread it on the MRS plate medium, place it in a constant temperature incubator at 37°C for 24 hours and count it. Calculate the bacterial concentration and record, repeat 3 times to get the average value. The growth curve of Lactobacillus johnsonii NJD412 was drawn with the culture time (hours) as the abscissa and the bacterial concentration...
Embodiment 3
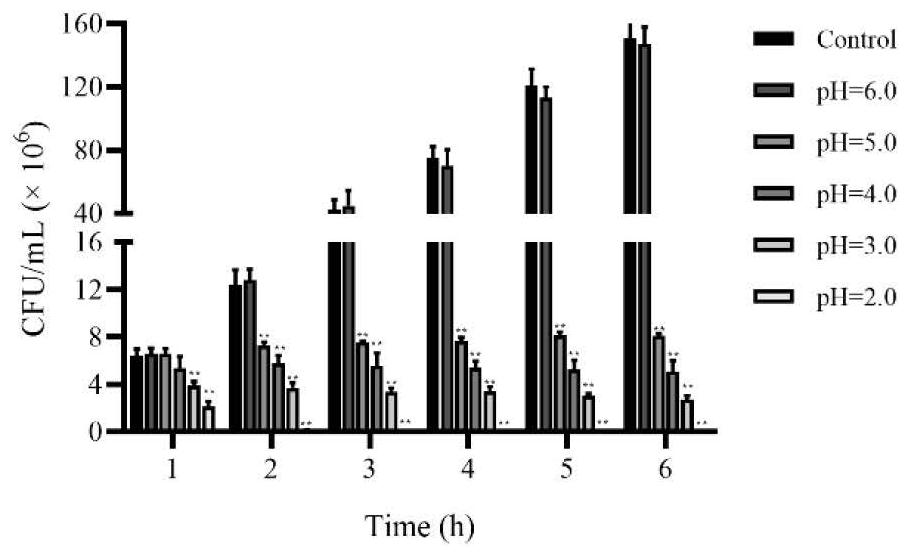
[0076] Embodiment 3-Inhibition test of Lactobacillus johnsonii NJD412 to pathogenic bacteria
[0077] In this example, the test of the bacteriostatic ability of Lactobacillus johnsonii NJD412 was carried out by taking enterotoxogenic Escherichia coli and Salmonella typhimurium as examples.
[0078] Lactobacillus johnsonii NJD412 was inoculated into MRS liquid medium at a proportion of 5%, and cultured at 37°C for 24h, and the concentration of the bacterial solution was adjusted to 1×10 8 CFU / mL. After the bacterial liquid was centrifuged at 4000 rpm for 10 min, and filtered with a bacterial filter with a diameter of 0.22 μm, the fermentation broth of Lactobacillus johnsonii NJD412 without bacterial cells was obtained.
[0079] Activated enterotoxogenic Escherichia coli and Salmonella typhimurium were evenly inoculated on LB plates (purchased from Qingdao Haibo Biological Co., Ltd., HB0129), inverted at 37°C for 1 hour, and then taken out. Circular holes were punched on the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com