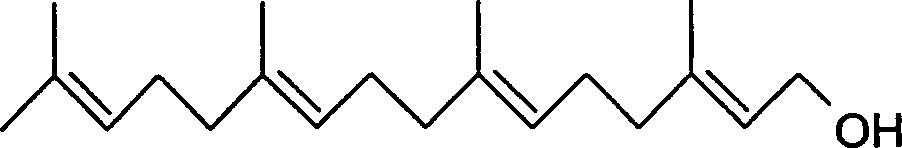
Synthesis of geranyl geraniol
A technology of geranylgeraniol and its synthesis method, which is applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., and can solve problems such as lack of practical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation of 9-sulfone-geranylgeraniol is obtained by condensation of trans-8-bromoacetic acid geranyl ester and geranyl sulfone under alkaline conditions. In this step, the α-H of the sulfone group in geranyl sulfone is taken away under strong alkaline conditions to form a carbanion, and then undergoes a nucleophilic substitution reaction with geranyl bromoacetate to generate the product 9-sulfone Geranylgeraniol.
[0028] The synthesis method proposed by Kikumasa Sato et al. uses n-butyllithium as a strong basic reagent in a mixed solvent of dimethylamide and hexamethylphosphoric triamide, and the reaction temperature is -78°C. Since n-butyllithium and hexamethylphosphoric triamide are very expensive, the present invention uses potassium tert-butoxide as a condensing agent in dimethylamide to carry out the condensation reaction at -20-0°C. Wherein the molar ratio of potassium tert-butoxide to geranyl sulfone is 1-2:1.
[0029] The sulfone group in the condensa...
Embodiment 2
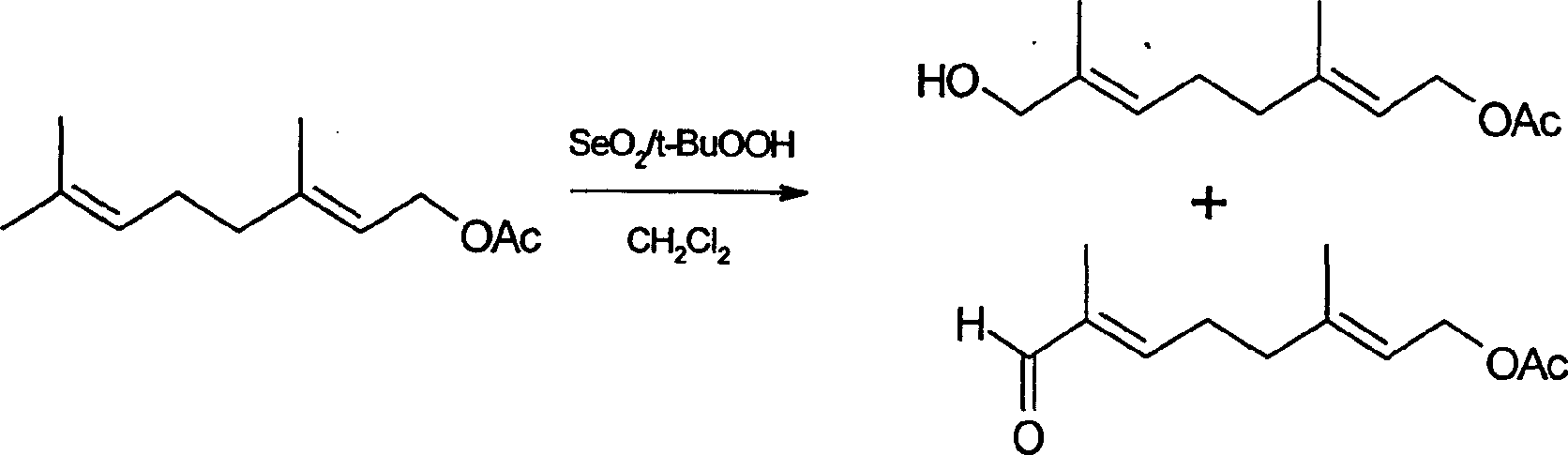
[0047] 1) Using geranyl acetate as a raw material, first in the solvent dichloromethane, use selenium dioxide-tert-butyl hydroperoxide binary oxidant at 0°C to selectively allyle the trans-methyl group of geranyl acetate Oxidation at the trans-allyl position to obtain a mixed oxidation product of trans-allyl carbonylation and hydroxylation; and then reduction with sodium borohydride in methanol at 0°C to obtain a single oxidation product, trans-geranyl 8-hydroxyacetate;
[0048] 2) Trans-geranyl 8-hydroxyacetate is reacted with phosphorus tribromide in anhydrous ether in the presence of pyridine at -15°C to obtain trans-geranyl 8-bromoacetate;
[0049] 3) Then in N,N-dimethylformamide, in the presence of potassium tert-butoxide, geranyl sulfone and trans-8-bromoacetic acid geranyl ester were condensed at -20°C to obtain 9-sulfone base aromatic Phyllogeraniol;
[0050] 4) 9-Sulfone-geranylgeraniol At -78°C, the sulfone group was removed using a lithium-methylamine reducing age...
Embodiment 3
[0052] 1) Using geranyl acetate as a raw material, first in the solvent dichloromethane, use selenium dioxide-tert-butyl hydroperoxide binary oxidant at 25°C to selectively allyle the trans-methyl group of geranyl acetate Oxidation at the trans-allyl position to obtain a mixed oxidation product of trans-allyl carbonylation and hydroxylation; then reduce it with sodium borohydride in methanol at 10°C to obtain a single oxidation product, trans-geranyl 8-hydroxyacetate;
[0053] 2) Trans-geranyl 8-hydroxyacetate is reacted with phosphorus tribromide in anhydrous ether in the presence of pyridine at -10°C to obtain trans-geranyl 8-bromoacetate;
[0054] 3) Then in N,N-dimethylformamide, in the presence of potassium tert-butoxide, geranyl sulfone and trans-8-bromoacetic acid geranyl ester were condensed at 0°C to obtain 9-sulfone-geranyl Geraniol;
[0055] 4) 9-Sulfone-geranylgeraniol At -60°C, the sulfone group was removed using a lithium-methylamine reducing agent to obtain the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com