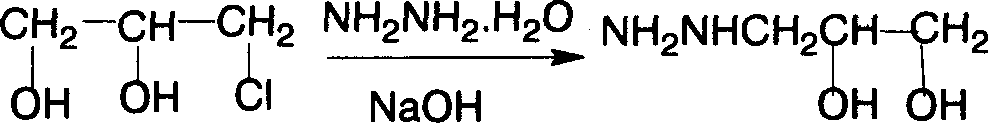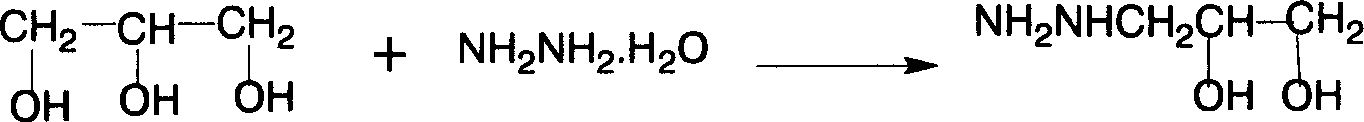Synthesis method of 5-hydroxy methyl furazolidone
A technology for furazolidone and a synthesis method, which is applied in directions such as organic chemistry, antibacterial drugs, etc., can solve problems such as excessively demanding process conditions, and achieve the effects of high product yield, easy industrialization, and few steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] First add 50g of 80% hydrazine hydrate aqueous solution, heat up to 80°C, add 27.5g of 1-chloroglycerin dropwise, after the addition, gradually raise the temperature to 120-125°C with reflux, react for 1 hour, then cool down to 40-45°C ℃, add 10.5g of sodium hydroxide, raise the temperature to 100 ℃, keep warm for 10 minutes, then, recover hydrazine hydrate by distillation under reduced pressure, distill to 110 ℃, vacuum 0.093Mpa, until no liquid evaporates, a large amount of solid sodium chloride appears, No need to handle, lower the temperature, and enter the next step of reaction;
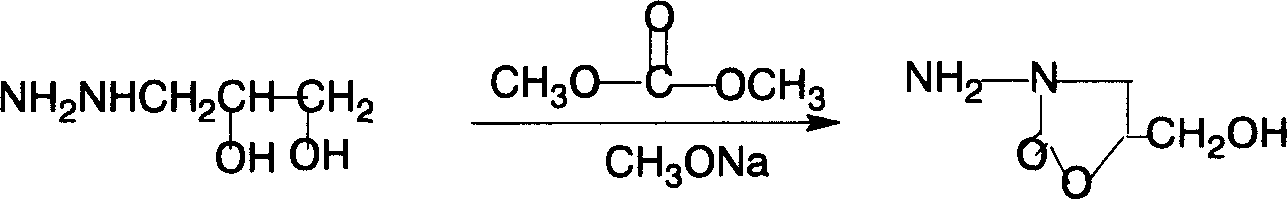
[0023] When the temperature drops to 70°C, add 9g of sodium methoxide, and after stirring for 5 minutes, add 25ml of dimethyl carbonate at 65°C, reflux at about 68°C for 30 minutes, recover methanol and dimethyl carbonate, add water to dissolve clear (water as solvent), add 80ml of hydrochloric acid (32%), heat to 65°C, add 26.5g of 5-nitrofurfural diacetate, heat to 84°C, keep warm for 2...
Embodiment 2
[0025] First add 200g of 20% hydrazine hydrate aqueous solution, heat up to 80°C, add 27.5g of 1-chloroglycerin dropwise, after adding, install a recovery device, gradually raise the temperature to above 100°C, recover part of the water until the temperature rises to 120- 125°C, change to reflux device, react for 1 hour, then cool down to 40-45°C, add 10.5g of sodium hydroxide, heat up to 100°C, keep warm for 10 minutes, then recover hydrazine hydrate by distillation under reduced pressure, distill to 110°C, Vacuum 0.093Mpa until no liquid is evaporated, and a large amount of solid sodium chloride appears, no need to deal with, lower the temperature, and enter the next step of reaction;
[0026] When the temperature drops to 70°C, add 9g of sodium methoxide, and after stirring for 5 minutes, add 25ml of dimethyl carbonate at 65°C, reflux at about 68°C for 30 minutes, recover methanol and dimethyl carbonate, add water to dissolve clear (water as solvent), add 80ml of hydrochlor...
Embodiment 3
[0028] First add 35g of 80% hydrazine hydrate aqueous solution, heat up to 90°C, add 18.5g of glycidol dropwise, and keep the temperature at 95-100°C. To 110 ° C, until no liquid evaporates, lower the temperature, and directly enter the next step of reaction;
[0029] When the temperature drops to 70°C, add 9g of sodium methoxide, and after stirring for 5 minutes, add 25ml of dimethyl carbonate at 65°C, reflux at about 68°C for 30 minutes, recover methanol and dimethyl carbonate, add water to dissolve clear (water as solvent), add 80ml of hydrochloric acid (32%), heat to 65°C, add 26.5g of 5-nitrofurfural diacetate, heat to 84°C, keep warm for 20 minutes, a yellow solid precipitates, cool to 40°C, and suction filter, After washing with water, washing with ethanol, and drying, 29.9 g of the product was obtained. The measured melting point was 240-242° C. (241-243° C. in literature). The purity by HPLC analysis was 98.7%, and the total yield was 42%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com