Method for preparing crystalline cefathiamidine and its usage
A cefathiamidine crystallization technology, which is applied in the field of crystalline cefathiamidine and its preparation, can solve the problems of inconvenient production operation, quality not conforming to cefathiamidine, high cost, etc., achieves reduction of production cost, simple and easy control of the production process, and clinical Apply a good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] At normal temperature, put 5 kg of cefathiamidine into the reactor, add 49 liters of water to dissolve, stir for 30 minutes, filter, adjust the pH of the filtrate to 4.5 with hydrochloric acid, add 200 liters of acetone until the filtrate is cloudy, and stir at 60 rpm , control the supersaturated concentration of the solution, continue to slowly add 200 liters of acetone / ethanol (weight ratio 10: 1), grow crystals, filter with suction, wash 2 times with 50 liters of acetone, drain, and dry under reduced pressure to obtain crystalline cefathiamidine 4.6 kilogram.
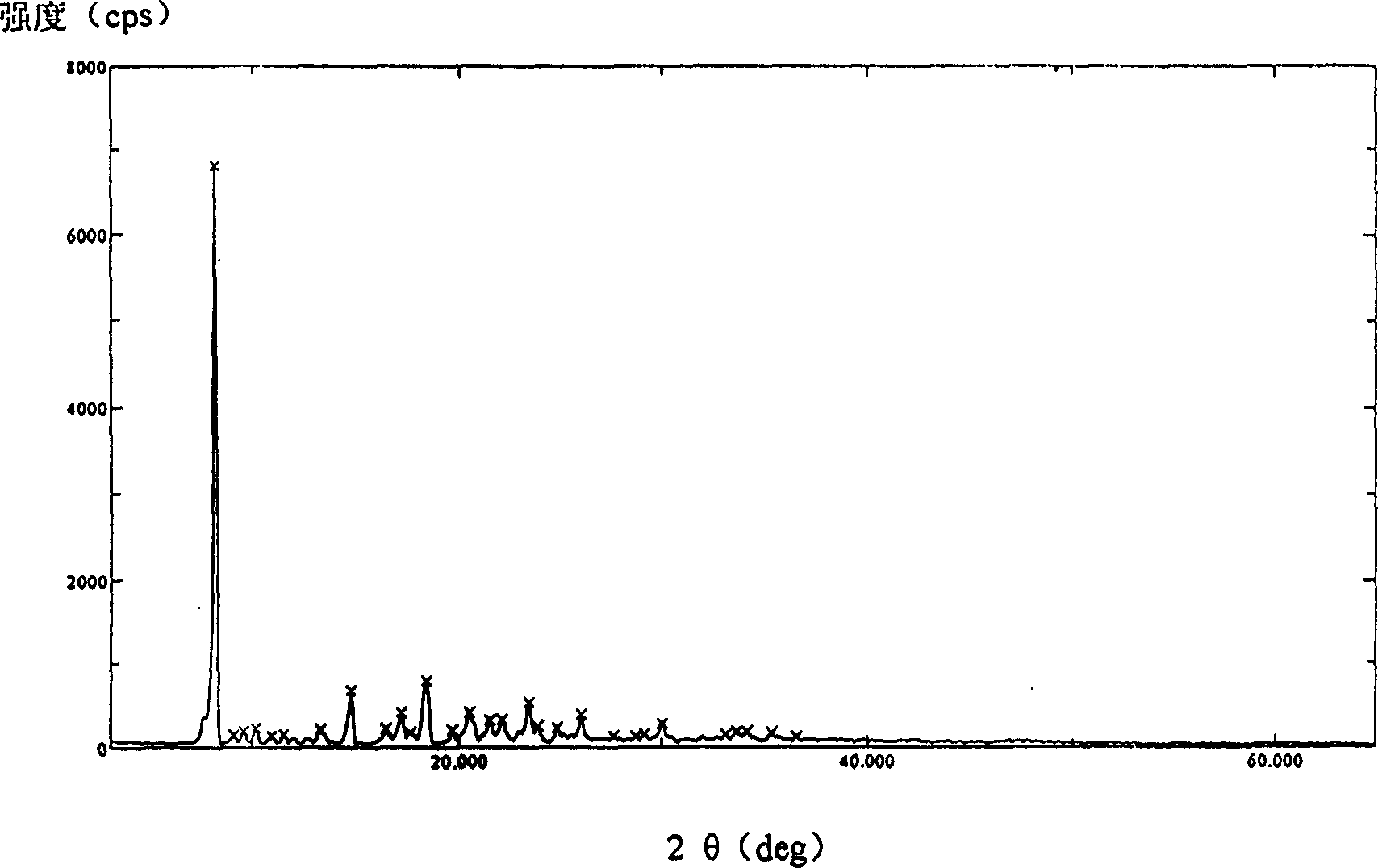
[0029]Use D / max-IIIA DIFFRATOMETER (RIGAKU CORPORATION, JANPAN) X-diffractometer, with Cu, K α 1, λ=1.54056A ray measurement, the X-ray powder diffraction pattern of crystalline cefathiamidine (see attached figure 1 ) is expressed in terms of 2θ, d-plane spacing and relative intensity greater than 5% as follows: 2θ d relative intensity I / I. (>5%)7.540 11.7146 58.340 ...
Embodiment 2
[0031] At 0° C., 50 liters of a 14% (w / w) cefathiamidine aqueous solution having a pH of 5.5 was put into the reactor, and sterile filtered. Under the aseptic production environment conditions that meet the requirements of GMP, with stirring at 70 rpm, add 300 liters of acetone to the sterile filtrate until the filtrate is cloudy, control the supersaturated concentration of the solution, continue to slowly add 350 liters of acetone, grow crystals, pump filtered, washed twice with 50 liters of acetone, drained, and dried under reduced pressure to obtain 7.3 kg of crystalline cefathiamidine sterile powder.
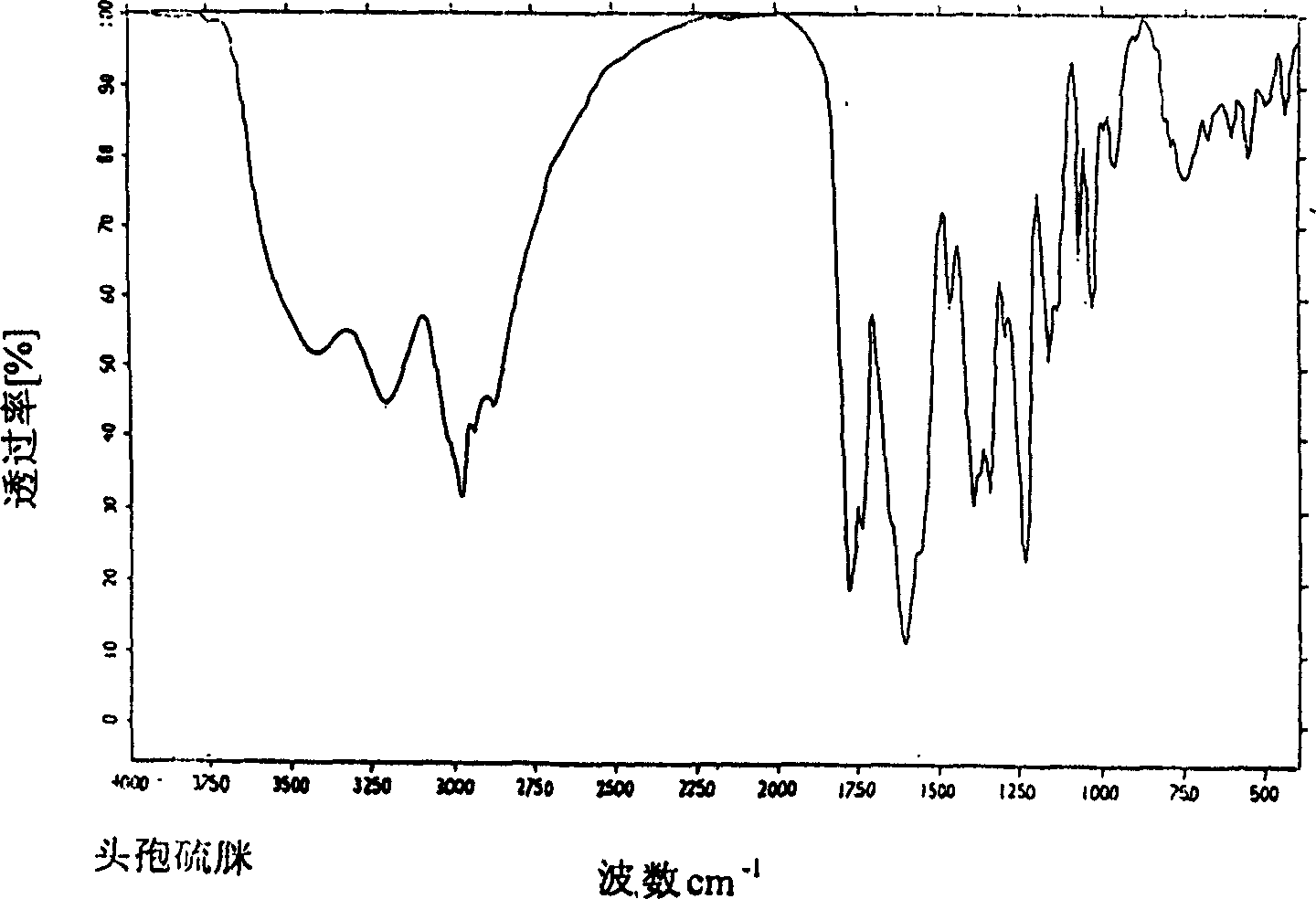
[0032] The X-ray and infrared spectra of the obtained crystals are consistent with those in Example 1. Stability test: according to the "Pharmacopoeia of the People's Republic of China" appendix drug stability test guidelines, select the amorphous cefathiamidine sample (batch number 8801) and the crystalline cefathiamidine sample (batch number 010210020) prepared by this emb...
Embodiment 4
[0040] Under normal temperature, put 5 kilograms of cefathiamidine crude products into the reactor, add 49 liters of water to dissolve, add 0.1 kilogram of activated carbon, stir for 30 minutes, press filter, adjust the pH of the filtrate to 5.5 with triethylamine, stir at 70 rpm Next, add 300 liters of ethanol until the solution is turbid, control the supersaturated concentration of the solution, continue to add 330 liters of isopropanol, grow crystals, filter with suction, wash twice with 60 liters of acetone, drain, and dry under reduced pressure to obtain crystalline cefathiamidine 4.4 kg.
[0041] The obtained crystalline infrared spectrum is consistent with that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com