Osmotic pump type controlled release preparation of kurarinone and its preparing method
A technology for controlled release preparations and matrine, which is applied in pharmaceutical formulations, antiviral agents, medical preparations containing active ingredients, etc., and can solve problems that are difficult to industrialize large-scale production, difficult to control the release rate, and product batch-to-batch differences. It can reduce toxic and side effects, facilitate administration and administration, and maintain a long time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
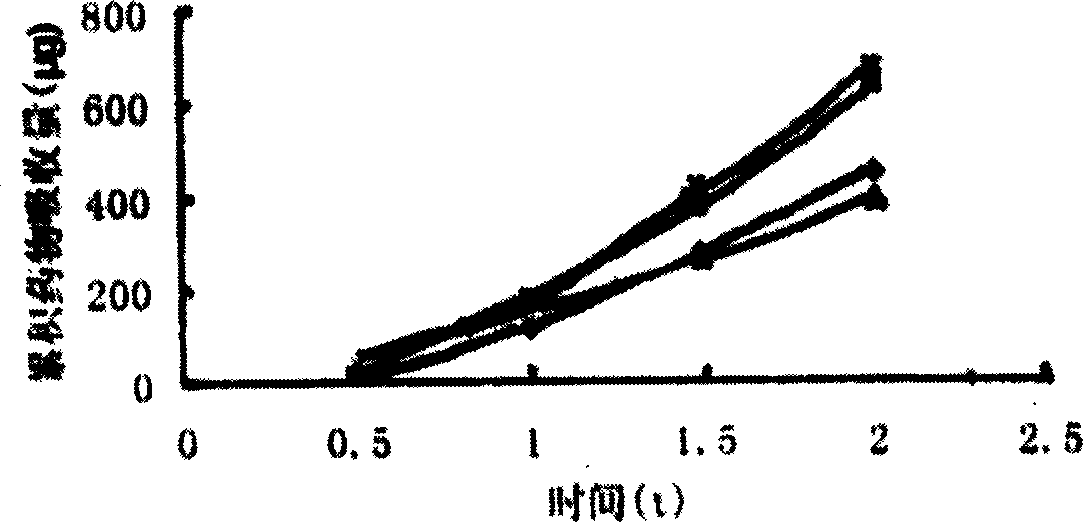
Image
Examples
Embodiment 1
[0030] Tablet Prescription:
[0031] Matrine 300.0g
[0033] Lactose 77.0g
[0035] Preparation of tablet cores: crush the prescribed amount of medicine, sodium chloride and lactose, sieve, mix evenly, use 95% ethanol as a wetting agent to make a soft material, granulate with a 20-mesh sieve, and dry the wet granules at 50±5°C. Sieve with 18 meshes, mix the dry granules with a lubricant, and press into tablets to obtain 1000 tablet cores.
[0036] Coating Solution Prescription:
[0037] Cellulose acetate 100g
[0038] PEG6000 12g
[0039] Dibutyl phthalate 13mL
[0040] Acetone 5L
[0041]Coating process: put the tablet core in the coating pan, blow in hot air, and coat the tablet core when the temperature is about 40°C. The flow rate of the coating solution is 5-10mL / min, and the pressure is 0.8kg / cm2 ,...
Embodiment 2
[0043] Tablet Prescription:
[0044] Matrine 300.0g
[0045] Sucrose 100.0g
[0046] Lactose 177.0g
[0048] Tablet core preparation, with embodiment 1:
[0049] Coating Solution Prescription:
[0050] Cellulose acetate 100g
[0051] PEG6000 12g
[0052] Dibutyl phthalate 5mL
[0053] Acetone 5L
[0054] Coating process, with embodiment 1.
Embodiment 3
[0056] Tablet Prescription:
[0057] Matrine 300.0g
[0059] Pregelatinized starch 77.0g
[0061] Tablet core preparation: same as in Example 1.
[0062] Coating liquid prescription: with embodiment 1.
[0063] Coating process: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com