Dispersion tablet of vasilowy hydrochlaride and its prepn. method
A technology for valacyclovir hydrochloride and dispersible tablets, applied in the field of antiviral drug valacyclovir hydrochloride dispersible tablets and the preparation thereof, can solve the problems of large single dose and the like, and achieves convenient administration, low production cost and high bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
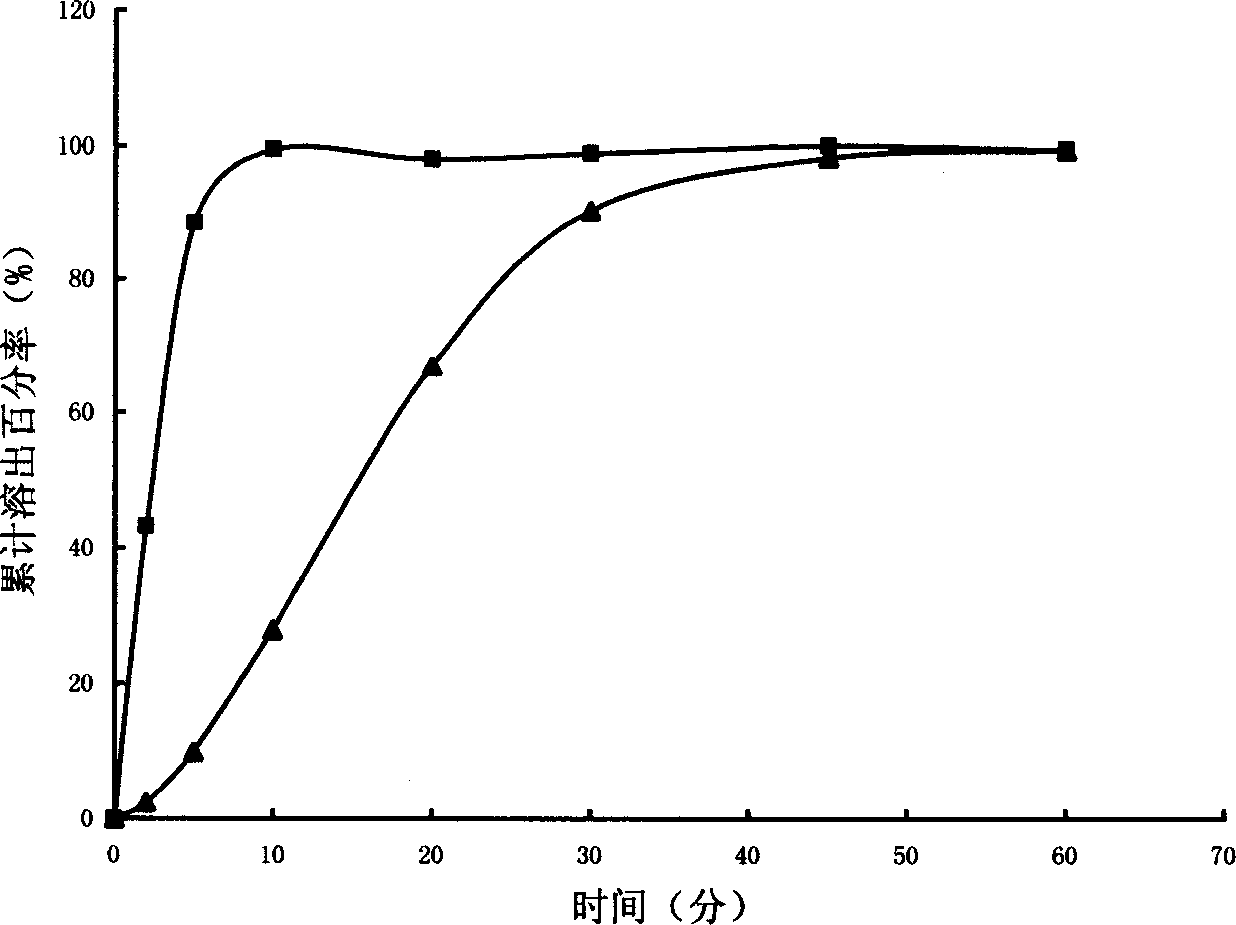
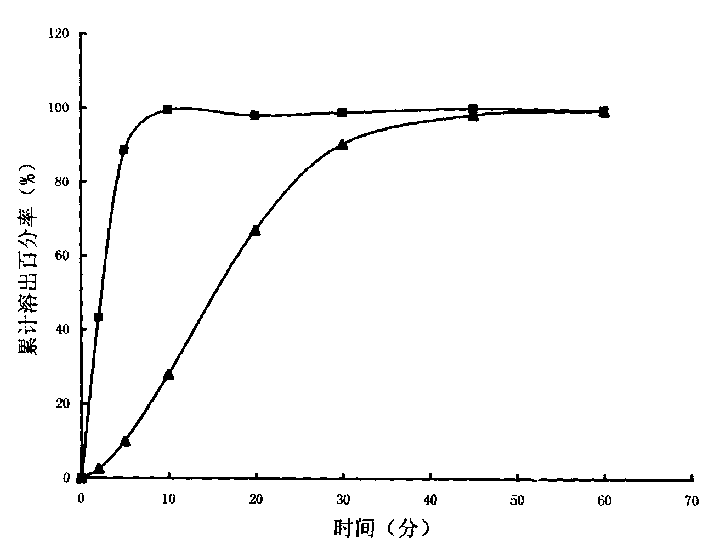
Image
Examples
Embodiment 1
[0014] Embodiment 1 per 1000 dosage
[0015] Valacyclovir Hydrochloride 200g
[0016] Microcrystalline Cellulose 530g
[0017] Hypromellose 40
[0018] Croscarmellose Sodium 66g (33g each inside and outside the grain)
[0019] Stevioside 40g
[0020] Polyvinylpyrrolidone (k30) 7g
[0021] Menthol 3g
[0024] Preparation of Valacyclovir Hydrochloride Dispersible Tablets
[0025] Take the prescribed amount of valacyclovir hydrochloride, microcrystalline cellulose, hydroxypropyl cellulose, stevioside and half the amount of croscarmellose sodium, sieve, mix well, and make polyvinylpyrrolidone into a 50 g / liter solvent Add the aqueous solution of the above mixture to make a soft material, pass through a 14-mesh sieve to granulate, dry at 50-60°C for 4 hours, add menthol, talcum powder, magnesium stearate and the remaining croscarmellose sodium, mix evenly, press Slice and serve.
Embodiment 2
[0026] Embodiment 2 per 1000 dosage
[0027] Valacyclovir Hydrochloride 500g
[0028] Microcrystalline Cellulose 200g
[0029] Starch 100g
[0030] Croscarmellose Sodium 70g
[0032] Hypromellose 2g
[0033] Talc powder 16g
[0035] Preparation of Valacyclovir Hydrochloride Dispersible Tablets
[0036] Get the prescribed amount of valacyclovir hydrochloride, microcrystalline cellulose, starch, croscarmellose sodium, and sodium saccharin, sieve, mix evenly, make hypromellose into a 5 g / liter aqueous solution, add the above The soft material is prepared from the mixture, granulated through a 24-mesh sieve, dried at 50-60°C for 4 hours, added with talcum powder and magnesium stearate, mixed evenly, and compressed into tablets.
Embodiment 3
[0037] Embodiment 3 per 1000 dosage
[0038] Valacyclovir Hydrochloride 500g
[0039] Microcrystalline Cellulose 700g
[0040] Cross-linked polyvinylpyrrolidone 64g (added outside the grain)
[0042] Polyvinylpyrrolidone (k30) 5g
[0043] Menthol 5g
[0044] Micronized silica gel 4g
[0046] Note: Polyvinylpyrrolidone is prepared as a 30 g / L solvent ethanol solution, and the solvent is a 50% volume ratio ethanol aqueous solution.
[0047] Preparation of Valacyclovir Hydrochloride Dispersible Tablets
[0048] Get the prescription amount of valacyclovir hydrochloride, microcrystalline cellulose, sodium saccharin, sieve, mix evenly, polyvinylpyrrolidone is made into the ethanol solution of 30 g / liter solvent with 50% ethanol by volume, add in the above-mentioned mixture to prepare The soft material is granulated through a 14-mesh sieve, dried at 50-60°C for 6 hours, added with menthol, micropowder silica gel, ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com