Porous calcium carbonate-hydroxyapatite gradient material and preparing method thereof
A technology of hydroxyapatite and porous calcium carbonate, which is applied in the field of medical materials, can solve problems such as non-degradability, and achieve the effects of simple production process, reduced production cost, and strict controllable conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The mechanism of water heat exchange reaction is (taking (NH4)2HPO4 as phosphate as an example):
[0036]
[0037] 10×100 6×136 2×18 1028 6×96 4×62
[0038] It can be seen that 1000 grams of CaCO3 is converted into Ca10(PO4)6(OH)2, (NH4)2HPO4816 grams are needed, therefore, by adjusting the reaction system (NH 4 ) 2 HPO 4 Gradient materials with different conversion ratios can be obtained.
[0039] Take by weighing (NH4) 2HPO4 4.08 grams (0.03mol) (sample 1), 8.16 grams (0.06mol) (sample 2), 16.32 grams (0.12mol) (sample 3), 32.64 grams (0.24mol) (test Sample 4), dissolved in triple-distilled water, reacted with 100 g (1 mol) of porous CaCO3 in a closed container at 280°C for 12 hours to obtain different samples, also weighed (NH4)2HPO4 and porous CaCO3, and reacted at 140°C After 96 hours, samples 5, 6, 7, and 8 were obtained. The samples were taken out, washed in boiling water, and dried for later use.
Embodiment 2
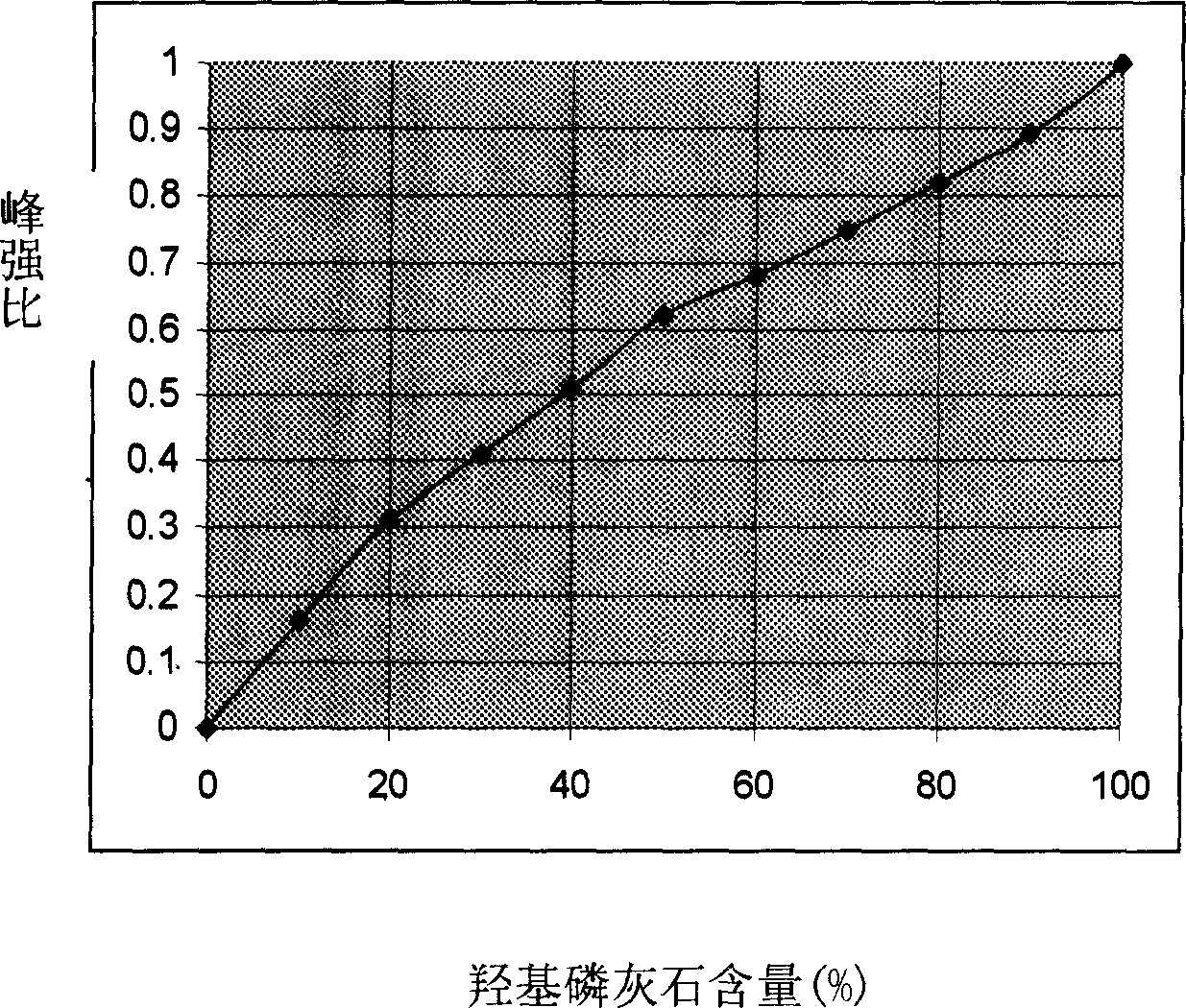
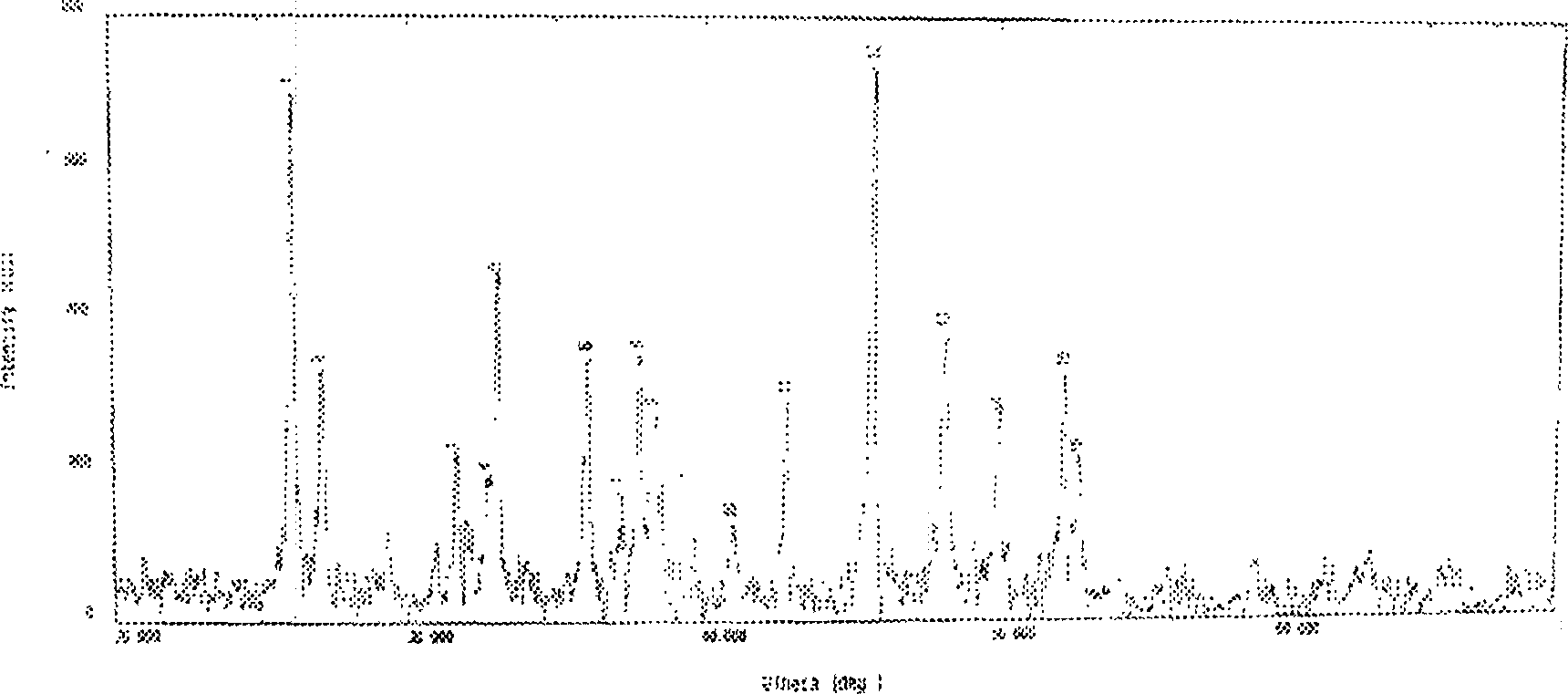
[0041] The coral calcium carbonate used in the experiment was ground into a fine powder, and mixed with the commercially available analytical pure hydroxyapatite powder at intervals of 10% (the ratios of hydroxyapatite and calcium carbonate were 100:0; 90:10; 80:20 ; 70:30; 60:40; 50:50; 40:60; 30:70; 20:80; 10:90; 0:100), X-ray diffraction analysis was carried out to obtain the X-ray diffraction peaks of the two Intensity ratio, draw the curve of hydroxyapatite content and peak intensity ratio ( figure 1 ). The sample obtained in Example 1 is ground into fine powder, dried, and X-ray diffraction analysis is carried out to determine its composition ( figure 2 ), and compared with the curve of hydroxyapatite content and peak intensity ratio, the content of hydroxyapatite in the sample was obtained (Table 1).
[0042] Table 1 is the curve of hydroxyapatite-calcium carbonate peak intensity ratio and hydroxyapatite content.
[0043] The abscissa is the hydroxyapatite content (...
Embodiment 3
[0062] The sample 2 sample obtained in embodiment 1, get section and carry out X-ray energy spectrum analysis, the result shows, sample surface ( image 3 Middle point A) The atomic ratio of calcium to phosphorus is 1.88 ( Figure 4 ),central( image 3 Middle B point) no phosphorus atom, still CaCO3 ( Figure 5 ). It shows that the hydroxyapatite formed after transformation covers the inner calcium carbonate in a thin coat. At the same time, the obtained product still maintains a good three-dimensional pore structure ( image 3 ).
[0063] image 3 This is the cross section of Sample 2 obtained in Example 1.
[0064] Figure 4 is the line spectrum analysis result of point A.
[0065] Figure 5 is the line spectrum analysis result of point B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com