Positive electrode active material and non-aqueous electrolyte secondary battery containing the same
A positive electrode active material and oxide technology, which is applied in the field of non-aqueous electrolyte secondary batteries, can solve the problems of reduced, unrealized, and difficult lithium-ion conductivity, and achieve excellent high-temperature characteristics, excellent lithium-ion conductivity, and excellent lithium-ion Effects of conductivity and high temperature characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
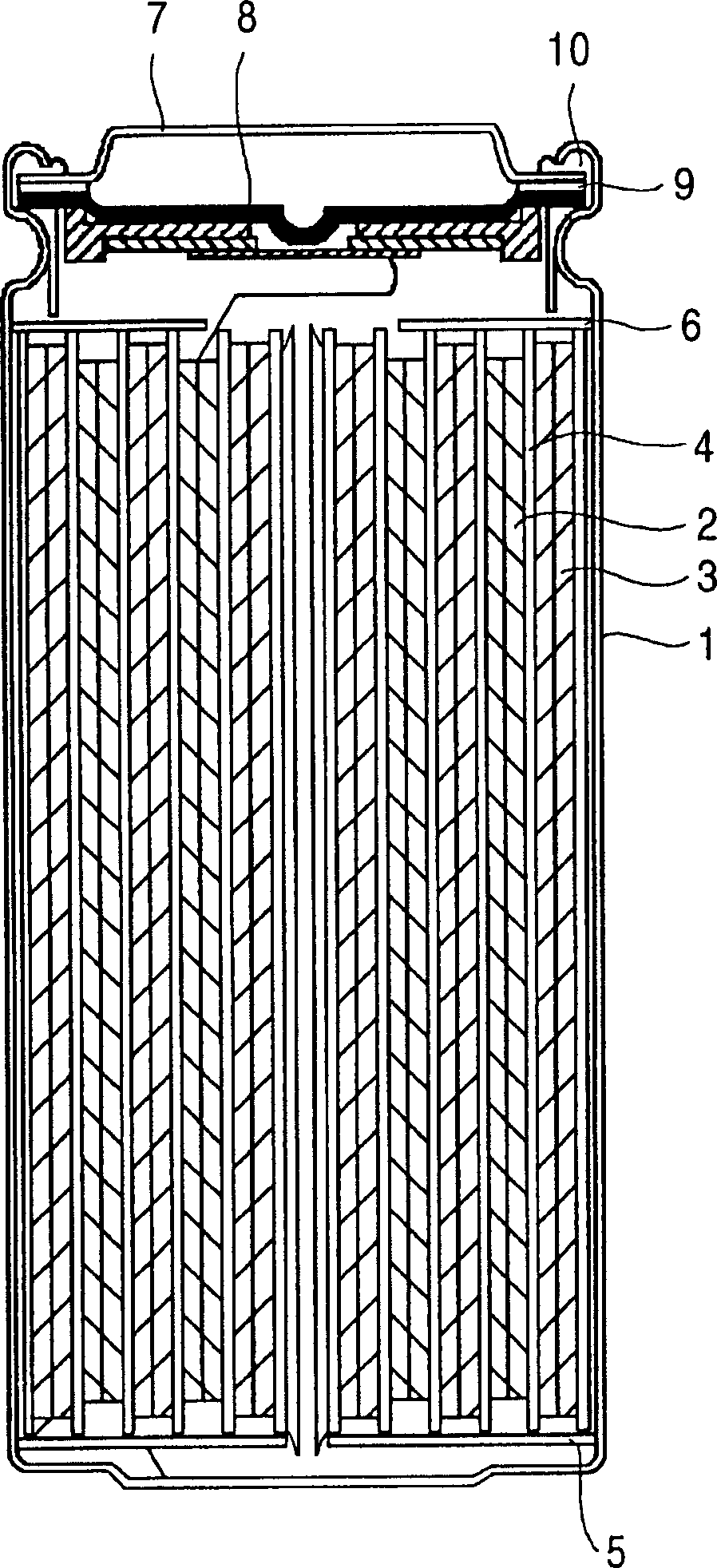
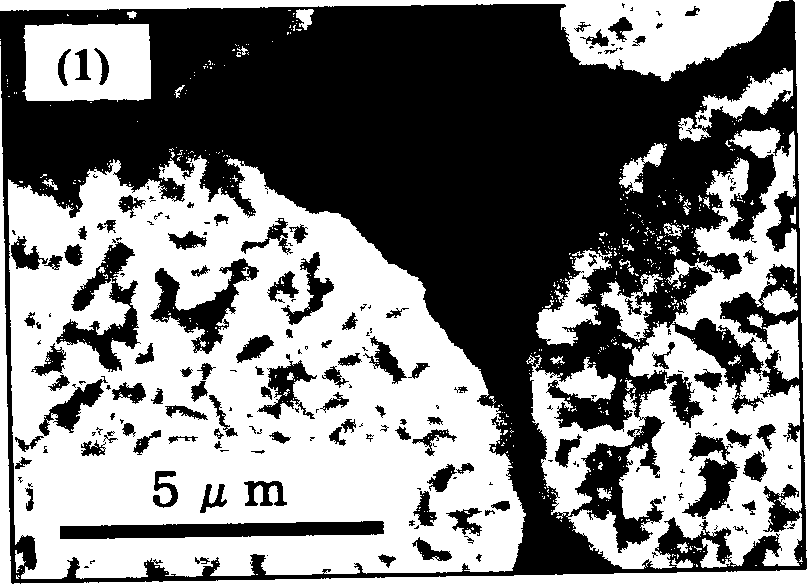
Image
Examples
preparation example Construction
[0029] The preparation method of the first composite oxide is not limited. For example, carbonates such as lithium carbonate and nickel carbonate are mixed according to components, and then the mixture is sintered at 600˜1100° C. in air or oxygen. Alternatively, a lithium raw material such as lithium hydroxide is mixed with a hydroxide prepared by co-precipitation of an aqueous inorganic salt solution mainly composed of nickel, and the mixture is sintered in air or oxygen. In addition, any other methods such as solid-phase synthesis and hydrothermal synthesis may be used as long as the above-mentioned properties can be achieved. Examples of raw materials include carbonate compounds, organic salts and oxides.
[0030] The above-mentioned second composite oxide can be produced by methods such as dry synthesis and hydrothermal synthesis disclosed in PCT Publication No. WO99 / 03784 and other known methods. Various lithium compounds and titanium compounds can be used as raw materi...
Embodiment 1
[0050] (Synthesis of the first composite oxide)
[0051] Commercially available nickel nitrate and manganese nitrate were mixed to obtain an aqueous solution at a ratio of Ni:Mn=0.70:0.30. Add ammonia water drop by drop and stir well to prepare composite hydroxide. This composite hydroxide was mixed with lithium hydroxide. The mixture was sintered at 850° C. in an oxygen flow for 10 hours, and then crushed to prepare lithium nickel manganese oxide. According to atomic absorption spectroscopy, the obtained powder has LiNi 0.70 mn 0.30 o 2 composition. According to the laser diffraction method, the powder had an average particle diameter of 13 μm. According to the X-ray diffraction method, its diffraction pattern is consistent with that of LiNiO in the International Center for Diffraction Data (ICDD) 09-0063 2 The graph is similar to that of LiNiO, that is, the graph shows that the powder has the same 2 Similar layered rock-salt structure. According to the detection of ...
Embodiment 2
[0063] Example 2 is prepared as in Example 1, except that in the preparation of the second composite oxide, the mixing ratio of the raw materials and the sintering temperature are changed to synthesize Li with a monoclinic crystal system 2 TiO 3 . Commercially available titanium oxide (anatase) and lithium hydroxide were mixed to obtain a Li:Ti=2:1 ratio mixture. The mixture was sintered at 950° C. in an oxygen flow for 12 hours, and then crushed to prepare lithium titanium oxide. According to the laser diffraction method, the powder had an average particle diameter of 0.6 μm. According to the method of X-ray diffraction, the diffraction pattern of the powder corresponds to that of ICDD 71-2348, which represents Li with a monoclinic crystal system 2 TiO 3 . Subsequently, a nonaqueous electrolyte secondary battery was prepared as in Example 1. The remaining capacity was measured at 45°C as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com