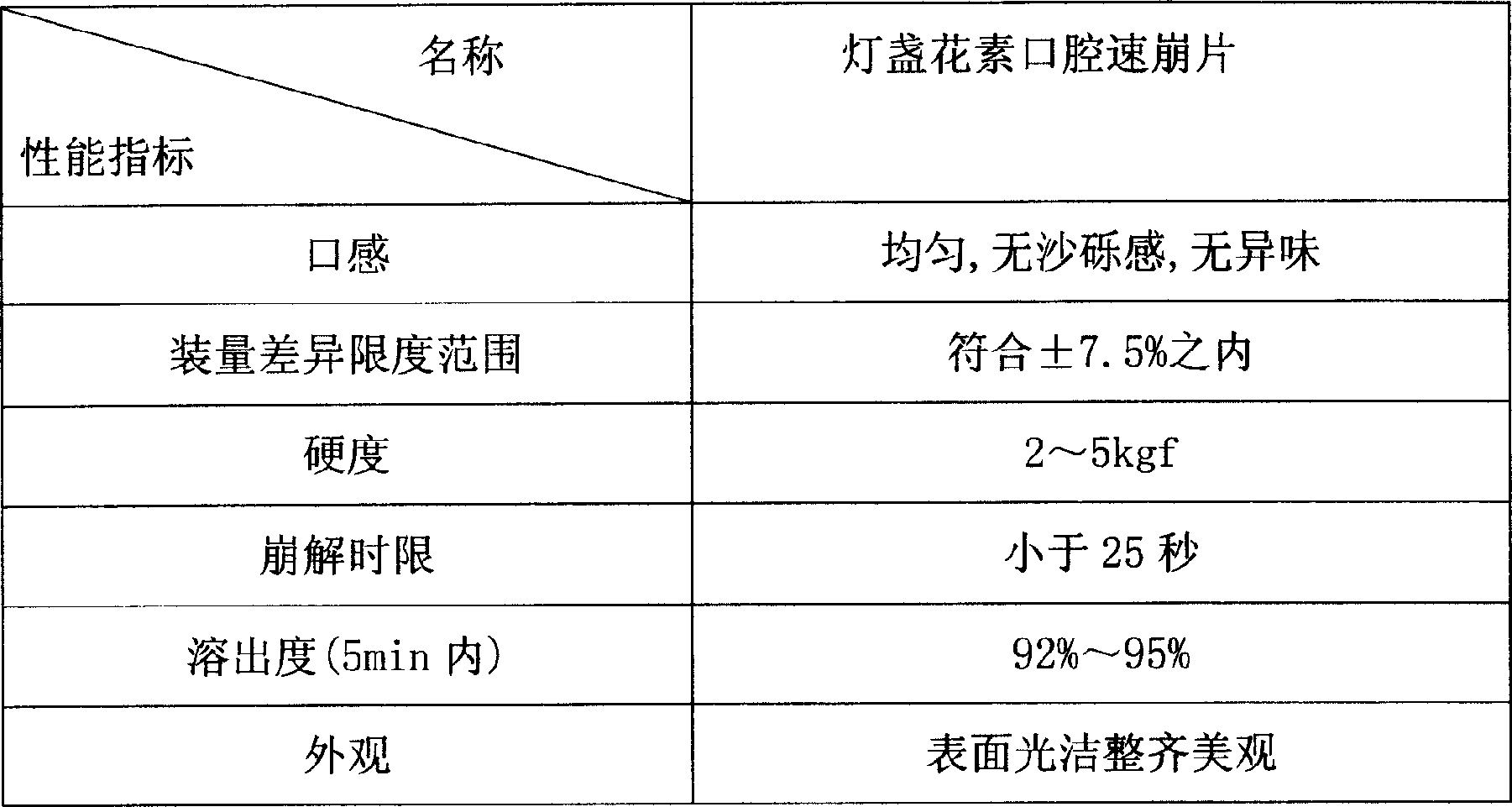
Breviscapine oral rapidly disintegrating formulation and its preparing process
A technology for breviscapine and oral fast disintegrating tablets, which is applied in the directions of pill delivery, pharmaceutical formulations, and medical preparations containing active ingredients, etc., can solve the problems of poor disintegration performance, shorten the drug dissolution time, etc. Good appearance, good absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] Example 1: Formula Breviscapine 20.10g
[0025] Microcrystalline Cellulose 45g
[0026] Low-substituted hydroxypropyl cellulose 5g
[0027] Micronized silica gel 2g
[0030] Starch 25g
[0031] Preparation method: Weigh the main drug and other auxiliary materials except magnesium stearate and 1 / 2 low-substituted hydroxypropyl cellulose according to the formula ratio, mix them thoroughly, pass through a 100-mesh sieve, and add an appropriate amount of distilled water to prepare a soft material , pass through a 20-mesh sieve to granulate, dry at 40-50°C, and sieve the granules and fine powder between 20-80 mesh, add the reserved low-substituted hydroxypropyl cellulose and magnesium stearate, mix well and press piece.
example 2
[0032] Example 2: Formula Breviscapine 20.10g
[0033] Microcrystalline Cellulose 60g
[0034] Low-substituted hydroxypropyl cellulose 6g
[0035] Micronized silica gel 2g
[0036] Magnesium stearate 0.5g
[0038] Starch 8.5g
[0039] Preparation method: Weigh the main drug and other auxiliary materials according to the formula ratio, pass through a 100-mesh sieve, fully mix, and directly compress the whole powder into tablets.
example 3
[0040] Example 3: Formula Breviscapine 23.0g
[0041] Microcrystalline Cellulose 54g
[0042] Low-substituted hydroxypropyl cellulose 5g
[0043] Micronized silica gel 1g
[0044] Magnesium stearate 0.5g
[0046] Starch 14.5g
[0047]Preparation method: Weigh the main drug and other auxiliary materials except magnesium stearate and 1 / 2 low-substituted hydroxypropyl cellulose according to the formula ratio, mix thoroughly, pass through a 100-mesh sieve, and add an appropriate amount of 60% ethanol to prepare Soft materials, granulated through a 20-mesh sieve, dried at 40-50°C, and granules and fine powders between 20-mesh and 80-mesh were sieved, added with low-substituted hydroxypropyl cellulose and magnesium stearate, and mixed well After compression.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com