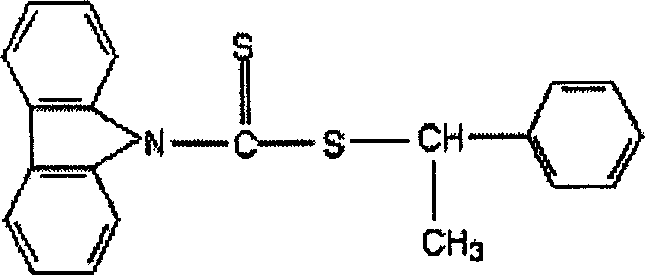
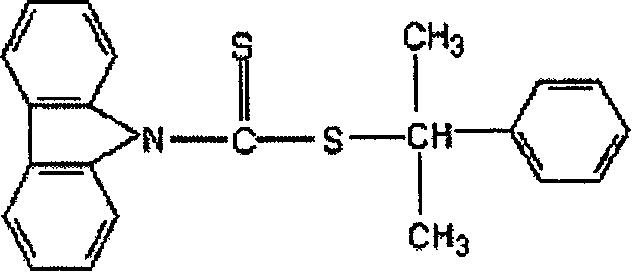
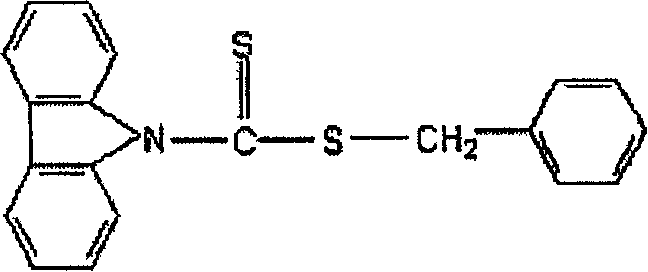Carbazyl dithio formate RAFT reagent and its preparation method and use
A technology of carbazole dithiocarboxylate and potassium carbazole dithiocarboxylate is applied in the field of living radical polymerization, carbazole dithiocarboxylate RAFT reagent and preparation, and can solve the problem of not being able to store and synthesize for a long time. Complex process, harsh reaction conditions and other problems, to achieve the effect of easy control, mild reaction conditions and good control ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0031] In a 100ml round bottom flask, add 3.34g (0.02mol) of carbazole and 40ml of tetrahydrofuran solvent, and then add 0.46g (0.02mol) of finely chopped metallic sodium. Under nitrogen protection, react in a water bath at 10° C. for 10 hours. The reaction was stopped and unreacted sodium was filtered off. Then add 1.52g (0.02mol) of carbon disulfide, and control the reaction temperature at 10°C for 4 hours. Sodium N-carbazole dithioformate is obtained.
example 2
[0033] In a 100ml round bottom flask, add 3.34g (0.02mol) carbazole and 40ml acetone solvent, then add 3.2g (0.08mol) sodium hydroxide. Under nitrogen protection, react in a water bath at 64° C. for 2 hours, stop the reaction, and filter out unreacted sodium hydroxide. Then add 6.08g (0.08mol) of carbon disulfide, and control the reaction temperature at 42°C for 1 hour. Sodium N-carbazole dithioformate is obtained.
example 3
[0035] In a 100ml round bottom flask, add 3.34g (0.02mol) carbazole and 40ml DMF solvent, then add 3.18g (0.03mol) sodium carbonate. Under nitrogen protection, react in a water bath at 55° C. for 5 hours, stop the reaction, and filter out unreacted sodium carbonate. Then add 3.04g (0.04mol) of carbon disulfide, and control the reaction temperature at 38°C for 2 hours. Sodium N-carbazole dithioformate is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com