Simple production process for zinc citrate, zinc lactate, ferrous citrate and ferrous lactate
A technology of ferrous lactate oral liquid and ferrous citrate, which is applied in the simple production process of ferrous citrate, ferrous lactate oral liquid, zinc citrate, and zinc lactate, can solve the problem of poor absorption efficiency and unstable ferrous , easy to be oxidized and other problems, to achieve the effect of good anti-oxidation performance, guaranteed purity and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
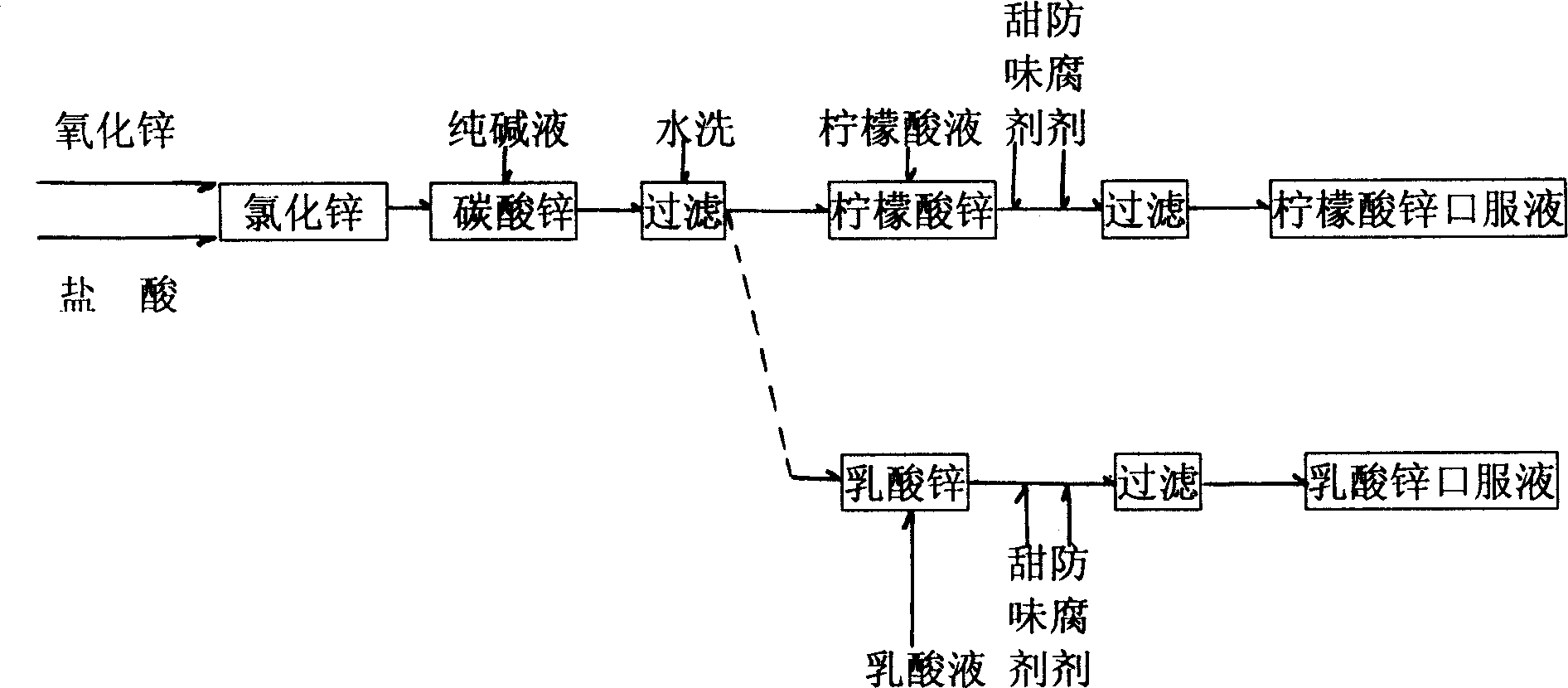
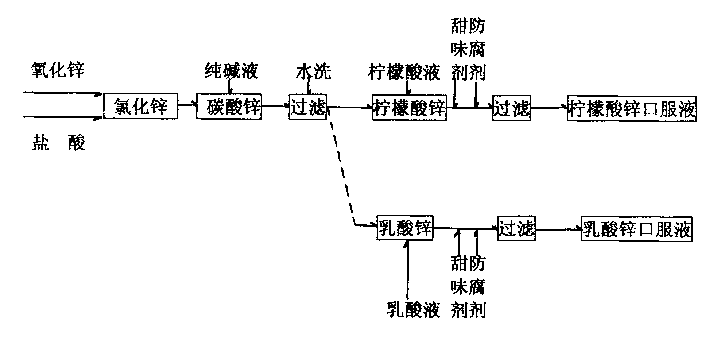
[0012] see figure 1 , use 1.2 kg of zinc oxide as raw material, add 2 kg of water, add 3 kg of hydrochloric acid or sulfuric acid with a concentration of 30%, and form zinc chloride or zinc sulfate through chemical reaction, make it fully soluble in the aqueous solution, and then add a concentration of 10% 20 kilograms of sodium carbonate solution, generate zinc carbonate precipitate through chemical displacement reaction, make solution final pH value be 8. Filter the sediment, put it into the reaction kettle that is dissolved in 27 kilograms of distilled water by 3 kilograms of solid edible citric acid and be diluted into the reaction kettle that concentration is 10% citric acid aqueous solution after washing twice with the water after the fine filter, in Fully stir at room temperature to form a zinc citrate solution, then add 950 kg of distilled water, and then add sweeteners and preservatives to obtain an oral solution of zinc citrate containing 10 mg of zinc per 10 ml. In...
Embodiment 2
[0016] see figure 1 , use 1.2 kg of zinc oxide as raw material, add 2 kg of water, add 3 kg of hydrochloric acid or sulfuric acid with a concentration of 30%, and form zinc chloride or zinc sulfate through chemical reaction, make it fully soluble in the aqueous solution, and then add a concentration of 10% 20 kilograms of sodium carbonate solution, generate zinc carbonate precipitate through chemical displacement reaction, make solution final pH value be 8. Filter the precipitate, wash it twice with finely filtered water, put it into a reaction kettle equipped with a 10% lactic acid aqueous solution diluted with 3 kg of lactic acid dissolved in 18 kg of distilled water, and fully Stir to form a zinc lactate solution, then add 950 kg of distilled water, and then add sweeteners and preservatives to obtain an oral solution of zinc lactate containing 10 mg of zinc per 10 ml, and finally pass the test and fill it into a 5-600 ml capacity In the bottle, a small measuring cylinder i...
Embodiment 3
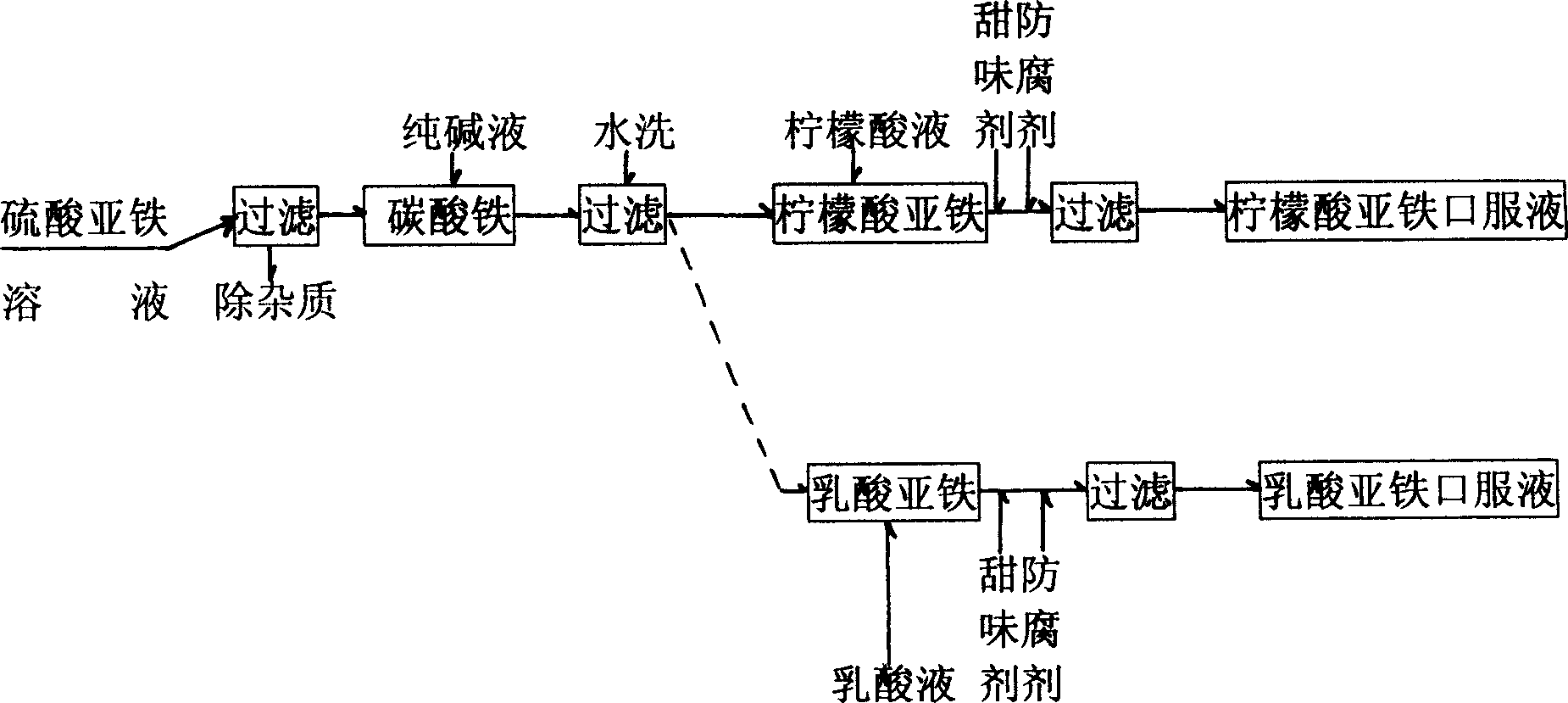
[0020] see figure 2 , use 7.5 kg of ferrous sulfate purified by filtration or recrystallization as raw material, add 100 kg of finely filtered water, stir well to fully dissolve ferrous sulfate, and then filter it into a closed reaction kettle , the upper part of the reactor was protected with carbon dioxide gas. Another 5 kg of 10% sodium carbonate solution made from edible sodium carbonate is added to the reaction kettle, and the iron carbonate precipitate is generated through stirring. Make the pH of the solution 10. Add the ferric carbonate precipitate after the above-mentioned pressure filtration in the reaction kettle diluted with 8 kilograms of solid edible citric acid and 72 kilograms of distilled water into a 10% citric acid solution, make the temperature gradually rise to 50 ° C, stir for 20 minutes, when When the solution pH value was 6, stop stirring and make ferrous citrate solution. Then add 170 kilograms of distilled water, then add sweetener and preservativ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com