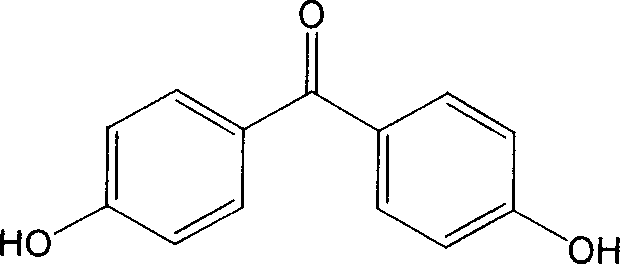
Method for preparing 4,4'-dihydroxy benzophenone
A technology of dihydroxybenzophenone and phenyl hydroxybenzoate, applied in 4 fields, can solve the problems of limited catalyst type, serious environmental pollution, difficult recovery of waste phosphoric acid, etc., and achieves reduction of production cost, simplified process and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] (1) p-acetoxybenzoic acid
[0017] Add p-hydroxybenzoic acid and acetic anhydride into a 250ml three-neck flask, stir and add a catalytic amount of concentrated sulfuric acid, heat at 65°C for 2 hours, pour the material into ice water, stir, precipitate solids, filter, and recrystallize ethanol to obtain a white solid p-Acetoxybenzoic acid, yield 93%, mp192-194.
[0018] (2) p-Acetoxybenzoyl chloride
[0019] Add 28g (0.16mol) of p-acetoxybenzoic acid, 40ml (0.55mol) of thionyl chloride and pyridine (0.06-0.1ml) into a three-necked flask equipped with a stirring and reflux condenser, and heat up to 70-75°C The reaction was stirred for 3h until no gas evolved. After cooling to room temperature, unreacted thionyl chloride was distilled off, and the residue was distilled under reduced pressure to collect fractions at 140-144°C / 10.67kPa to obtain 29g (0.15mol) of light yellow oily liquid p-acetoxybenzoyl chloride.
[0020] (3) Phenyl p-acetoxybenzoate
[0021] Add 15g (...
Embodiment 2
[0027] The preparation methods of p-acetoxybenzoic acid, p-acetoxybenzoyl chloride, and phenyl p-hydroxybenzoate are the same as in Example 1.
[0028] Using chlorobenzene as the solvent instead, reflux reaction for 10 hours to prepare phenyl p-acetoxybenzoate with a yield of 96%.
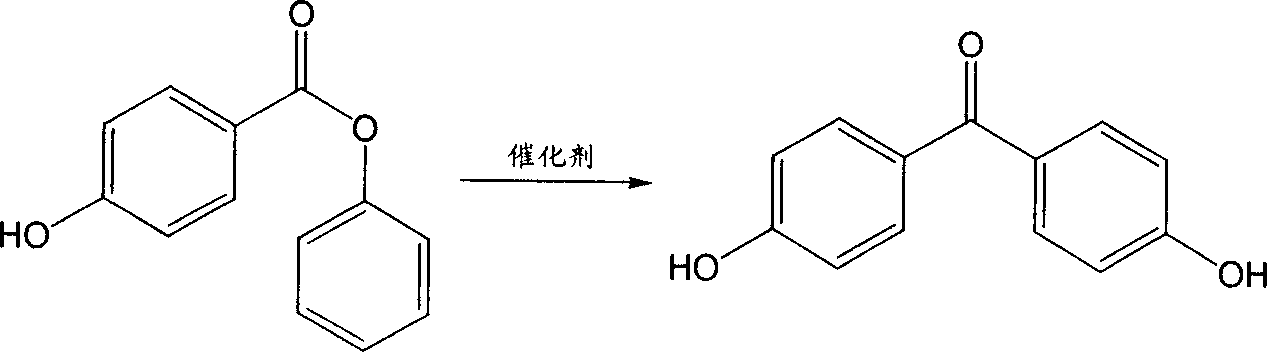
[0029] The ratio of phenyl p-hydroxybenzoate:trifluoromethanesulfonic acid is 1:0.2 (molar ratio), petroleum ether is used as solvent, and the temperature is raised, stirred and refluxed for 10 hours. Wash with 10% sodium bicarbonate solution and water, and recrystallize from methanol-water (1:3, volume ratio) to obtain the target compound 4,4'-p-dihydroxybenzophenone. Yield 98%. mp219~220℃.
Embodiment 3
[0031] The preparation methods of p-acetoxybenzoic acid, p-acetoxybenzoyl chloride, and phenyl p-hydroxybenzoate are the same as in Example 1.
[0032] Using chlorobenzene as the solvent instead, reflux reaction for 10 hours to prepare phenyl p-acetoxybenzoate with a yield of 96%.
[0033] Phenyl p-hydroxybenzoate: chromium trifluoromethanesulfonate at a ratio of 1:0.15 (molar ratio), cyclohexane as a solvent, and the temperature was raised to stir and reflux for 8 hours. Wash with 10% sodium bicarbonate solution and water, and recrystallize from methanol-water (1:3, volume ratio) to obtain the target compound 4,4'-p-dihydroxybenzophenone. Yield 99%. mp219~220℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com