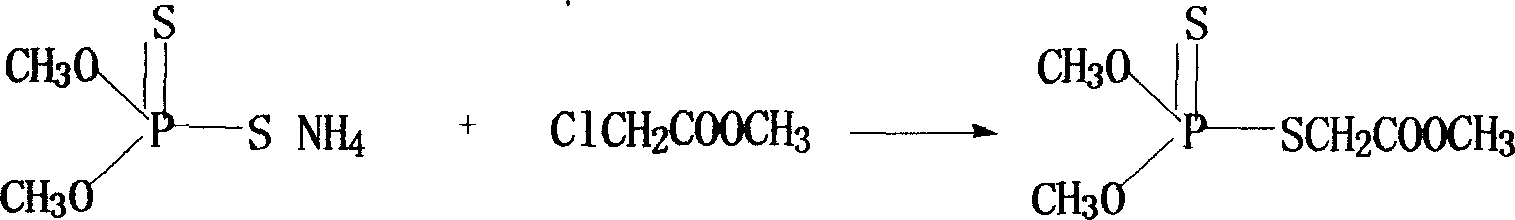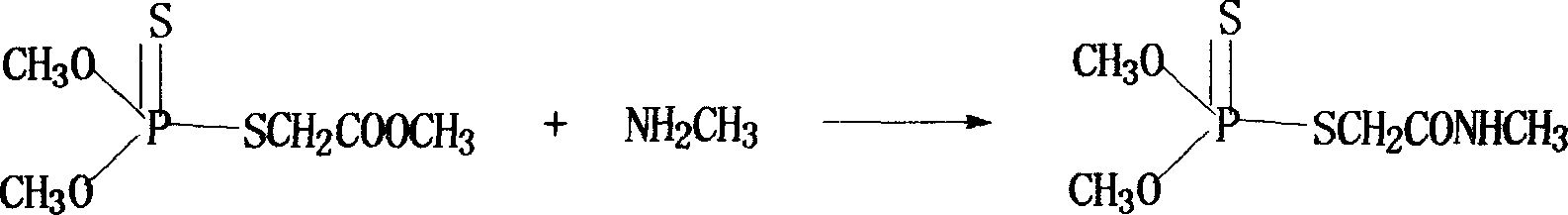Process for synthesizing dimethoate
A synthetic method and technology of dimethoate, applied in the field of dimethoate synthesis, can solve the problems of unreported dimethoate synthesis method, insufficient yield and purity, low toxicity, etc., and achieve remarkable social benefits, high income, and small investment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
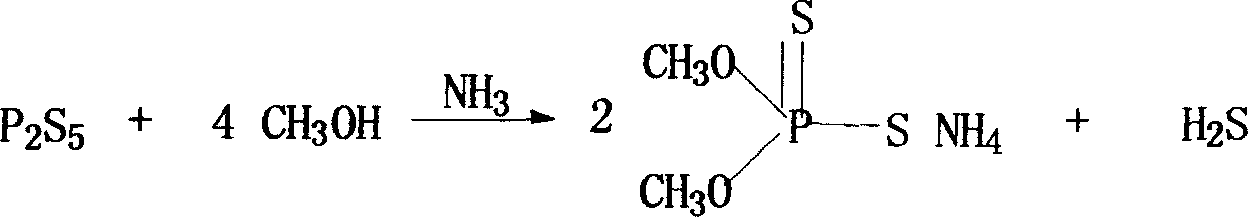
[0027] (1) The preparation of the intermediate phosphorothioate of dimethoate
[0028] Reaction formula:
[0029]
[0030] M=NH 4 , Na, K, Li
[0031] Prepare 1 mol of phosphorothioate into a 38% organic solution, such as methanol, ethanol, isopropanol or tetrahydrofuran, and put it into a reaction tank with 1 to 4 mol of methyl chloroacetate, start stirring, heat up to 0 to 60°C, and React in phase, keep warm for 80-120 minutes, then filter and remove solvent to obtain phosphorothioate.
[0032] (2) Dimethoate is synthesized from phospholipid and methylamine as raw materials
[0033] Reaction formula:
[0034]
[0035] Brief description of the process:
[0036] Put 1 mol of phosphorothioate into the tank, under the condition of -10~5℃, add a solution containing 1.0~1.4mol of monomethylamine, such as methanol, ethanol, acetone, isopropanol or tetrahydrofuran, and a solution that can dissolve phosphoric acid Add the organic solvent of salt to the reaction system. Af...
Embodiment 1
[0038] Add 18.44 grams of 38% ammonium salt methanol solution into the reaction tank, start stirring, and when heated to 55±5°C, add 8.68 grams of methyl chloroacetate dropwise. ~2 hours. Generate 8.38 g of phosphorothioate.
[0039] Cool the prepared phosphorothioate to -10-0°C, add dropwise a methanol solution containing 1.13 g of monomethylamine under stirring to carry out aminolysis. During the aminolysis process, keep warm for 75 to 150 minutes. Remove solvent, obtain dimethoate pure product 8.17 grams. The total yield is 88.9%.
Embodiment 2
[0041] Add 20.57 grams of 35% sodium salt methanol solution into the reaction tank, start stirring, and when heated to 55±5°C, add 8.68 grams of methyl chloroacetate dropwise. ~2 hours. Generate 8.42 g of phosphorothioate.
[0042] The prepared phosphorothioate was cooled to -10-0°C, and a methanol solution containing 1.13 g of monomethylamine was added dropwise with stirring to carry out aminolysis. During the aminolysis process, keep warm for 75 to 150 minutes. Remove solvent, obtain dimethoate pure product 8.02 grams. The total yield is 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com