An enzyme linked immuno-detection kit suitable for furazolidone retention analysis and application
An enzyme-linked immunosorbent assay and furazolidone technology, applied in the field of immunochemical analysis, can solve problems such as no patent reports on furazolidone ELISA kits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1, the preparation of antigen, antibody
[0022] 1.1 Synthesis of artificial antigen (immunogen, coater)
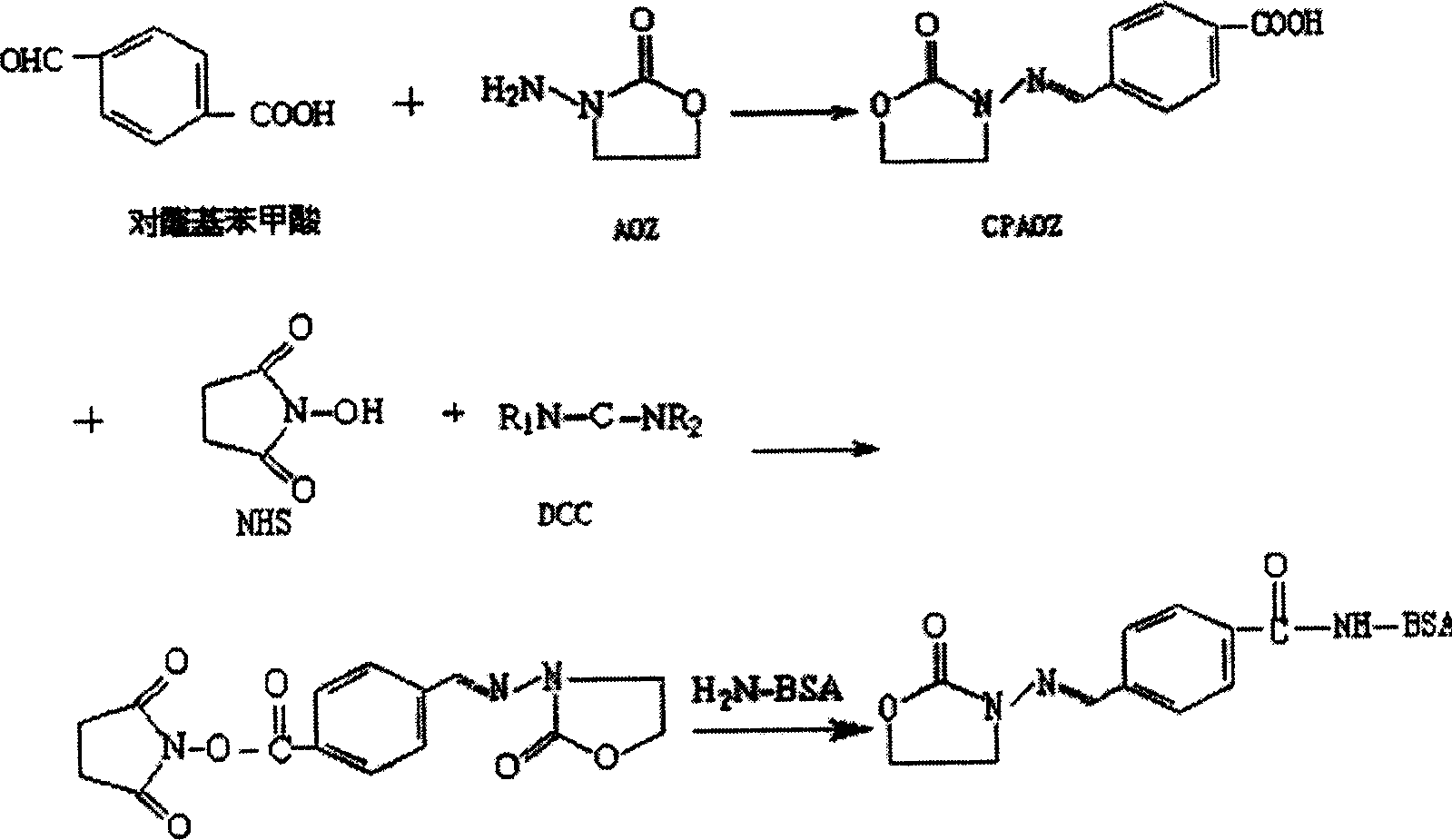
[0023] Add 10 mL of distilled water and 3.0 g of p-aldehyde benzoic acid into a 100 mL beaker with a magnetic stirrer device, slowly add N, N-dimethylformamide (DMF) dropwise until the p-aldehyde benzoic acid is completely dissolved, and add AOZ while stirring 1.0 g, filtered after reacting for 2 hours, washed with water three times to obtain 3-(4-carboxybenzylidene)-amino-2-oxazolidinone (abbreviated as CPAOZ, the same below) as the reactant of AOZ and p-formylbenzoic acid. Dissolve 23.4 mg of CPAOZ in 2 mL of DMF, add 27.5 mg of dicyclohexylcarbodiimide (DCC) and 14.4 mg of N-hydroxysuccinimide (NHS) while stirring, and react with magnetic stirring at 4°C overnight. After centrifugation, the supernatant is liquid A. Weigh 170 mg of bovine serum albumin (BSA) and dissolve it in 10 mL of 0.1 mol / L PBS (pH 8.0), add 1 mL of DMF, stir and dissolve to pre...
Embodiment 2
[0027] Embodiment 2, establishment of furazolidone indirect competitive detection method
[0028] 2.1 Determination of the optimal coating concentration of antigen and the optimal working concentration of antibody
[0029] Determined by the square matrix titration test, the point where the absorbance value is about 1.0 and the difference between the left and right adjacent absorbance values is the largest is taken as the reference point. Operation steps: Coat the first row of the 96-well ELISA plate with 2000 μg / L of the coating agent, and the second to eighth rows are sequentially coated with 1000, 500, 250, 120, 60, 30, 15 μg / L of the original Be original. Overnight at 4°C, block with 1% ovalbumin at 37°C for 1 hour, wash twice, pat dry, add 100 μL to the 1st to 9th column of the microtiter plate in sequence, the dilution factor is 10000, 20000, 40000, 80000, 160000, For 320000, 640000, 1280000, 2560000 3-amino-2-oxazolidinone antibodies, add sample diluent to the 10th c...
Embodiment 3
[0039] Example 3, the preparation of the enzyme-linked immunoassay kit for furazolidone residue analysis of the present invention
[0040] 3.1 Composition of the enzyme-linked immunoassay kit of the present invention
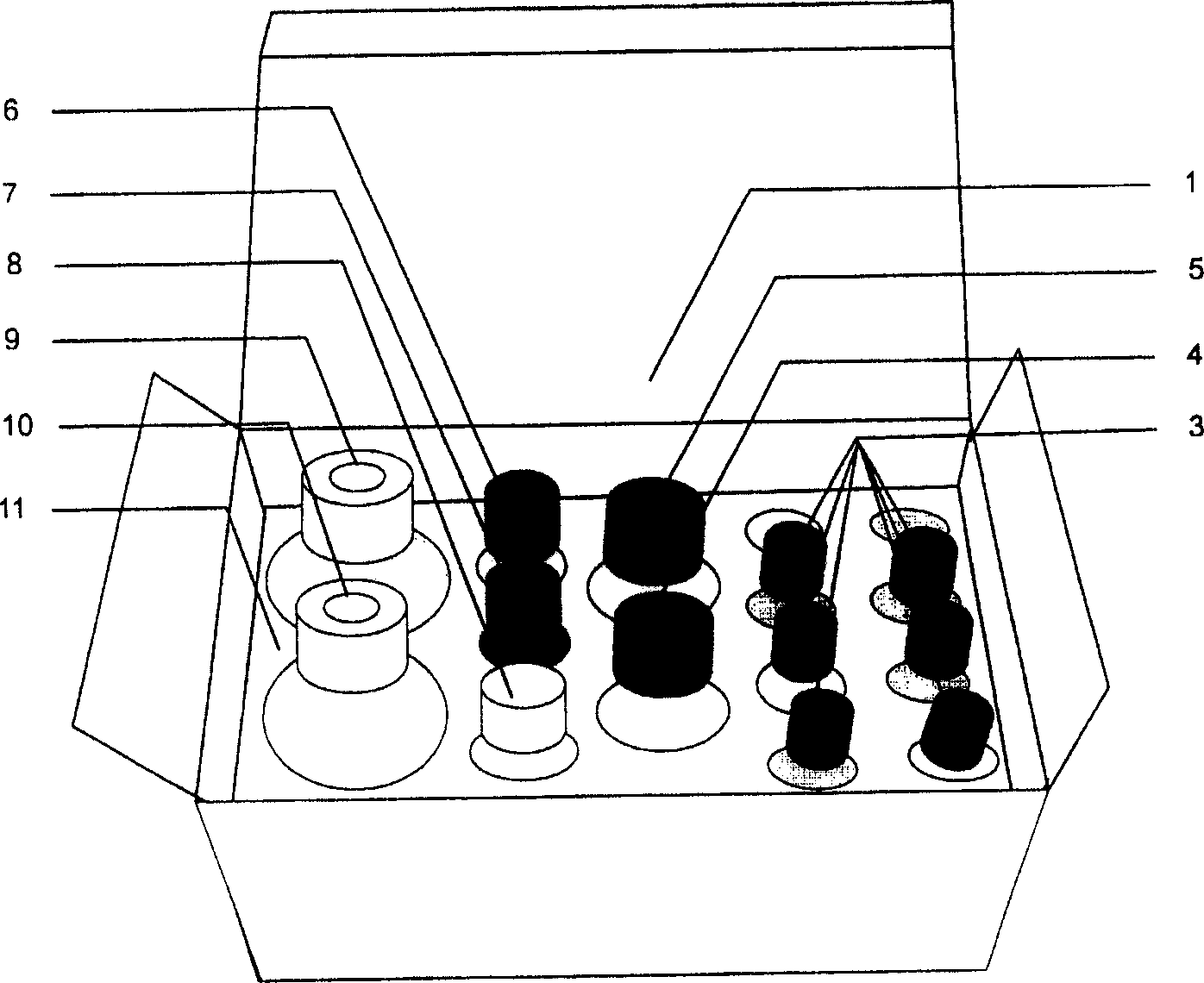
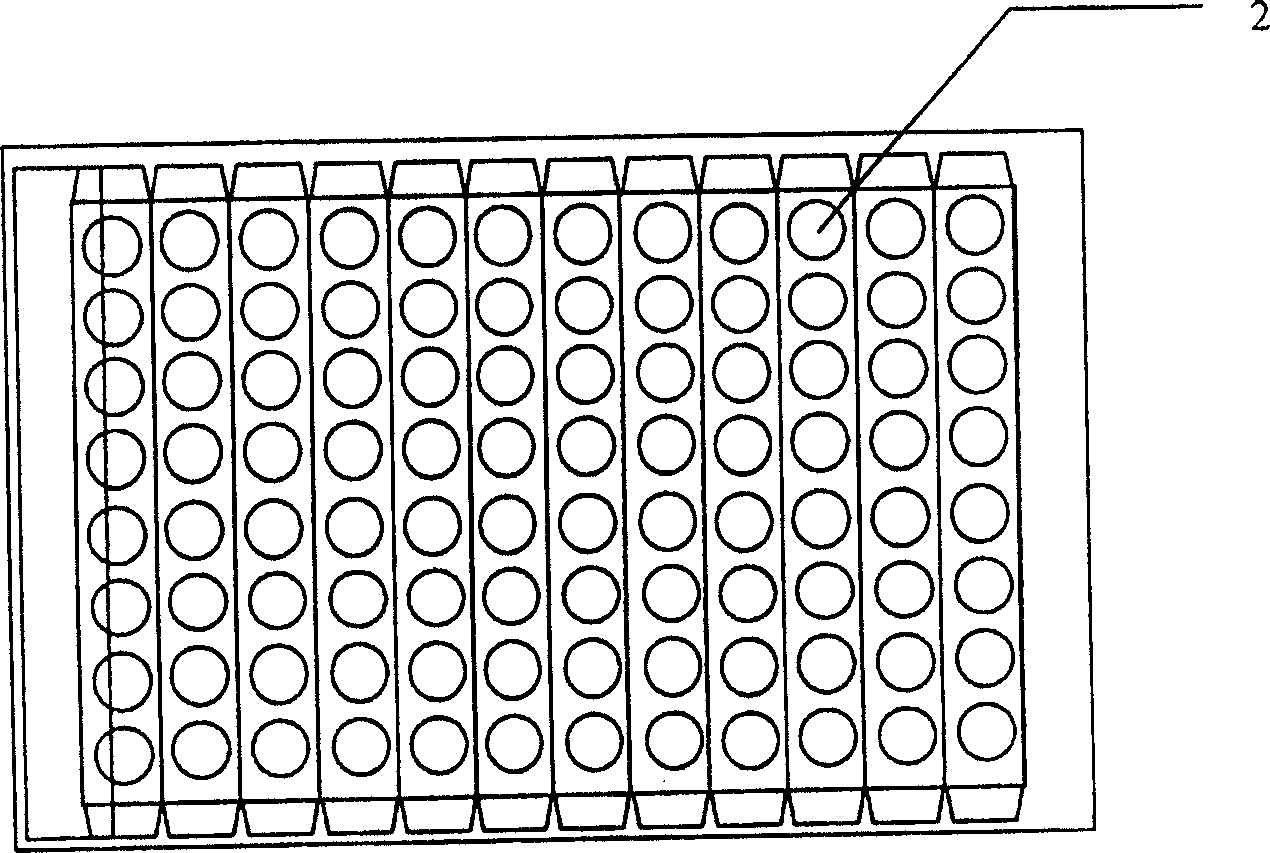
[0041] The kit of the present invention is mainly composed of a box body (1), an ELISA plate (2), 6 bottles of AOZ standard solution (3), horseradish peroxidase (HRP)-labeled goat anti-rabbit antibody (4), AOZ antibody Solution (5), Substrate Color Development Solution A (6), Substrate Color Development Solution B (7), Stop Solution (8), Wash Solution (9), Sample Diluent Solution (10) and Foam Holder (11) composition.
[0042] 3.2 Preparation of reagents
[0043] Prepared by conventional methods: where coating diluent: Na 2 CO 3 1.5g, NaHCO 3 2.9g, Na 2 N 3 0.2g, add double distilled water to 1000mL, adjust to pH9.6; blocking solution: ovalbumin 0.1g dissolved in pH7.4PBS 100mL; washing solution: NaCl 8.0g, KH 2 PO 4 0.2g, Na 2 HPO 4 12H 2 O 2.9g, KC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com