Method for preparing mica iron oxide by hydrothermal reaction and crystallizing
A technology of micaceous iron oxide and hydrothermal reaction, which is applied in the direction of iron oxide, iron oxide/iron hydroxide, fibrous filler, etc., can solve the synergistic effect and regulation effect of boric acid usage, mica iron oxide particle size and color Poor control effect, increased difficulty and load of impurity ions, etc., to achieve the effect of easy industrial application, strong brightness and three-dimensional effect, and smooth crystal surface
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
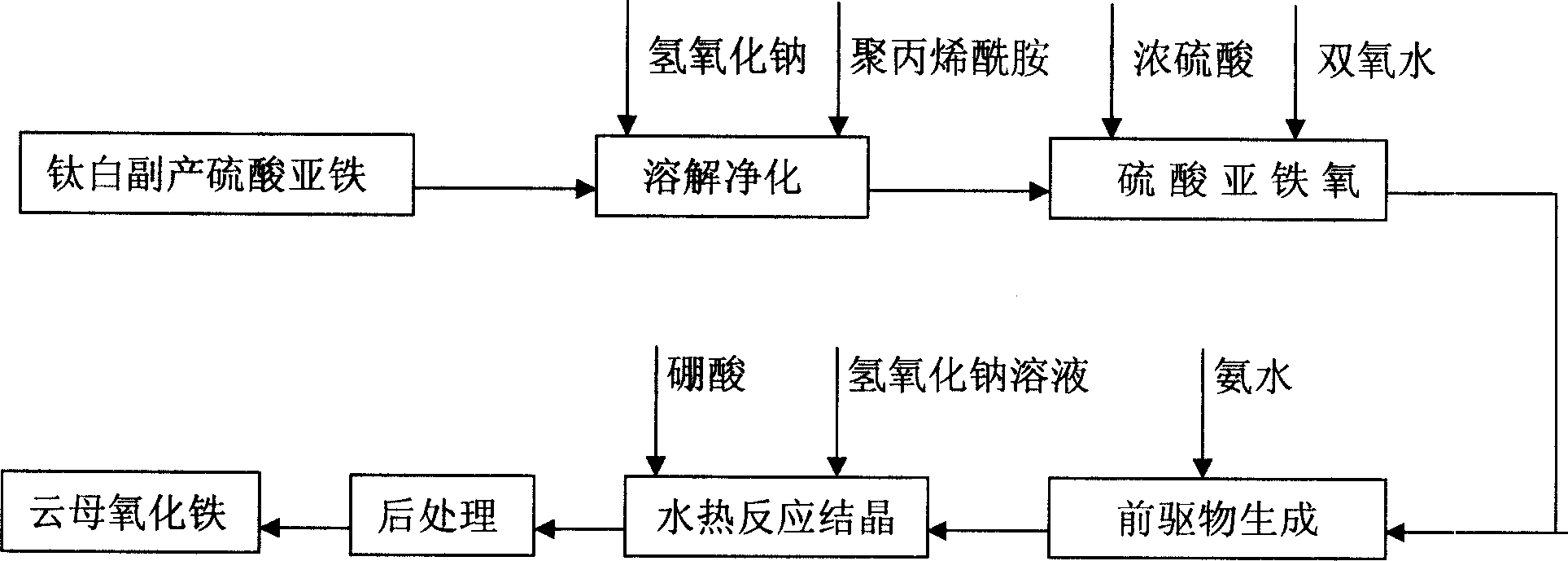
[0038] Dissolve 80g of titanium dioxide by-product ferrous sulfate in 300ml of soft water with a temperature of 60°C, filter, and the filtrate is the once-purified ferrous sulfate solution. Add 1.5g of NaOH precipitant and 2.0g of 20% polyacrylamide flocculant, stir slowly for 0.5h, then let it stand for 3h, the supernatant is the secondary purified ferrous sulfate solution. At a temperature of 60°C, 14g of concentrated sulfuric acid with a concentration of 98% was added to the ferrous sulfate solution for secondary purification, and then 23g of hydrogen peroxide with a concentration of 27.5% was added to make the FeSO 4 Oxidized to Fe 2 (SO 4 ) 3 , cooled to room temperature. In the solution after the above reaction, drip 115g concentration of ammonia water of 25%, while stirring, to make Fe 2 (SO 4 ) 3 Fe(OH) transformed into slurry 3 Precursor, stand for 1h, decant. The resulting slurry was washed to pH 6.0. Transfer the slurry to an autoclave with a volume of 1L, ...
Embodiment 2
[0040] Dissolve 80g of titanium dioxide by-product ferrous sulfate in 300ml of soft water with a temperature of 60°C, filter, and the filtrate is the once-purified ferrous sulfate solution. Add 1.5g of NaOH precipitant and 2.0g of 20% polyacrylamide flocculant, stir slowly for 0.5h, then let it stand for 3h, the supernatant is the secondary purified ferrous sulfate solution. At a temperature of 60°C, 14g of concentrated sulfuric acid with a concentration of 98% was added to the ferrous sulfate solution for secondary purification, and then 23g of hydrogen peroxide with a concentration of 27.5% was added to make the FeSO 4 Oxidized to Fe 2 (SO 4 ) 3 , cooled to room temperature. In the solution after the above reaction, drip 115g concentration of ammonia water of 25%, while stirring, to make Fe 2 (SO 4 ) 3 Fe(OH) transformed into slurry 3 Precursor, stand for 1h, decant. The resulting slurry was washed to pH 6.0. Transfer the slurry to a 1L autoclave, add a NaOH solutio...
Embodiment 3
[0042] Dissolve 80g of titanium dioxide by-product ferrous sulfate in 300ml of soft water with a temperature of 60°C, filter, and the filtrate is the once-purified ferrous sulfate solution. Add 1.5g of NaOH precipitant and 2.0g of 20% polyacrylamide flocculant, stir slowly for 0.5h, then let it stand for 3h, the supernatant is the secondary purified ferrous sulfate solution. At a temperature of 60°C, 14g of concentrated sulfuric acid with a concentration of 98% was added to the ferrous sulfate solution for secondary purification, and then 23g of hydrogen peroxide with a concentration of 27.5% was added to make the FeSO 4 Oxidized to Fe 2 (SO 4 ) 3 , cooled to room temperature. In the solution after the above reaction, drip 115g concentration of ammonia water of 25%, while stirring, to make Fe 2 (SO 4 ) 3 Fe(OH) transformed into slurry 3 Precursor, stand for 1h, decant. The resulting slurry was washed to pH 6.0. Transfer the slurry to a 1L autoclave, add a NaOH solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com