Sustained-release microsphere of LHRH antagonist for injection and preparation process thereof
A technology of sustained-release microspheres and antagonists, which can be applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and drug combinations, etc., and can solve the problems of patient pain, low bioavailability, and short biological half-life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Preparation of LXT-101 Microspheres by Double Emulsion Method
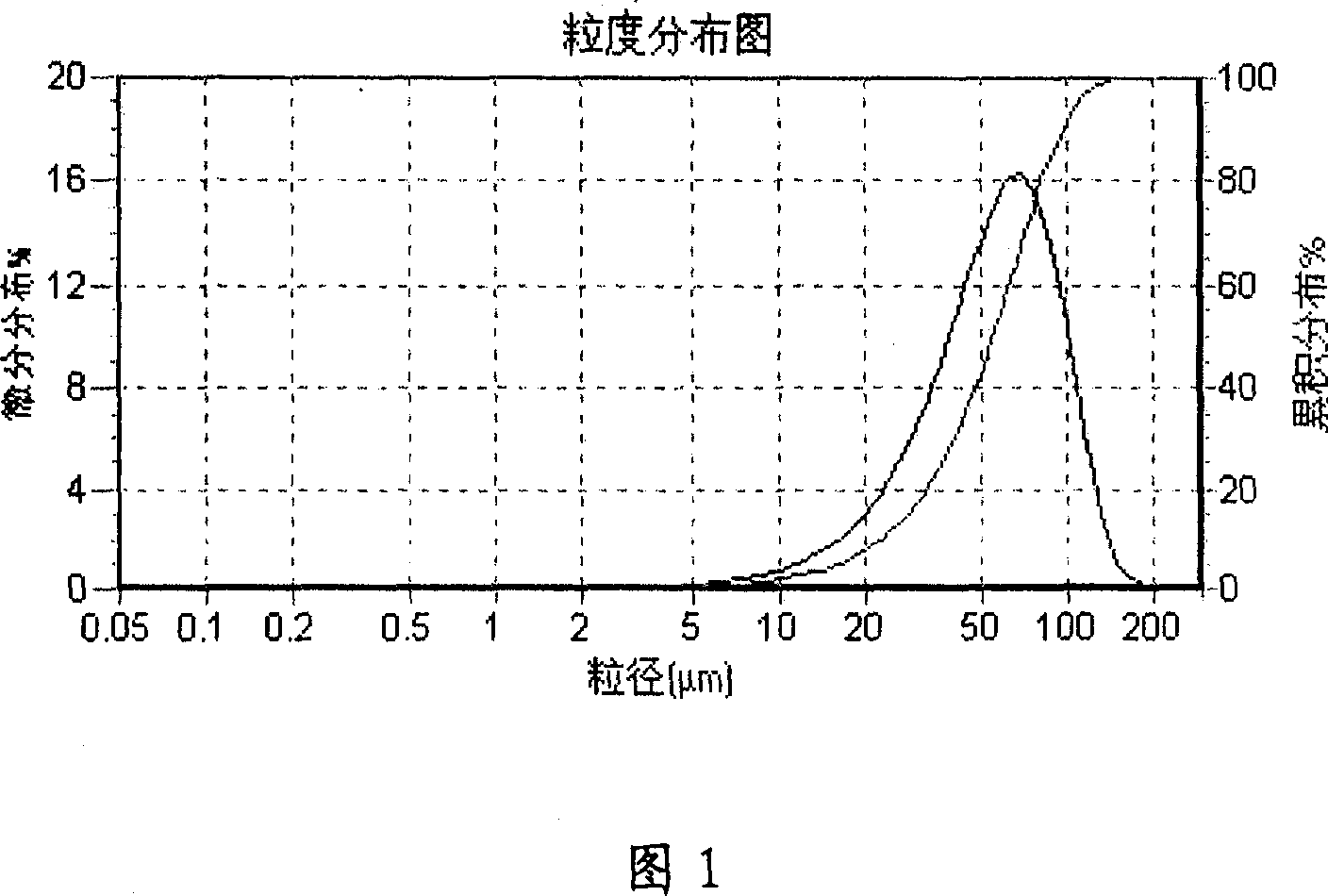
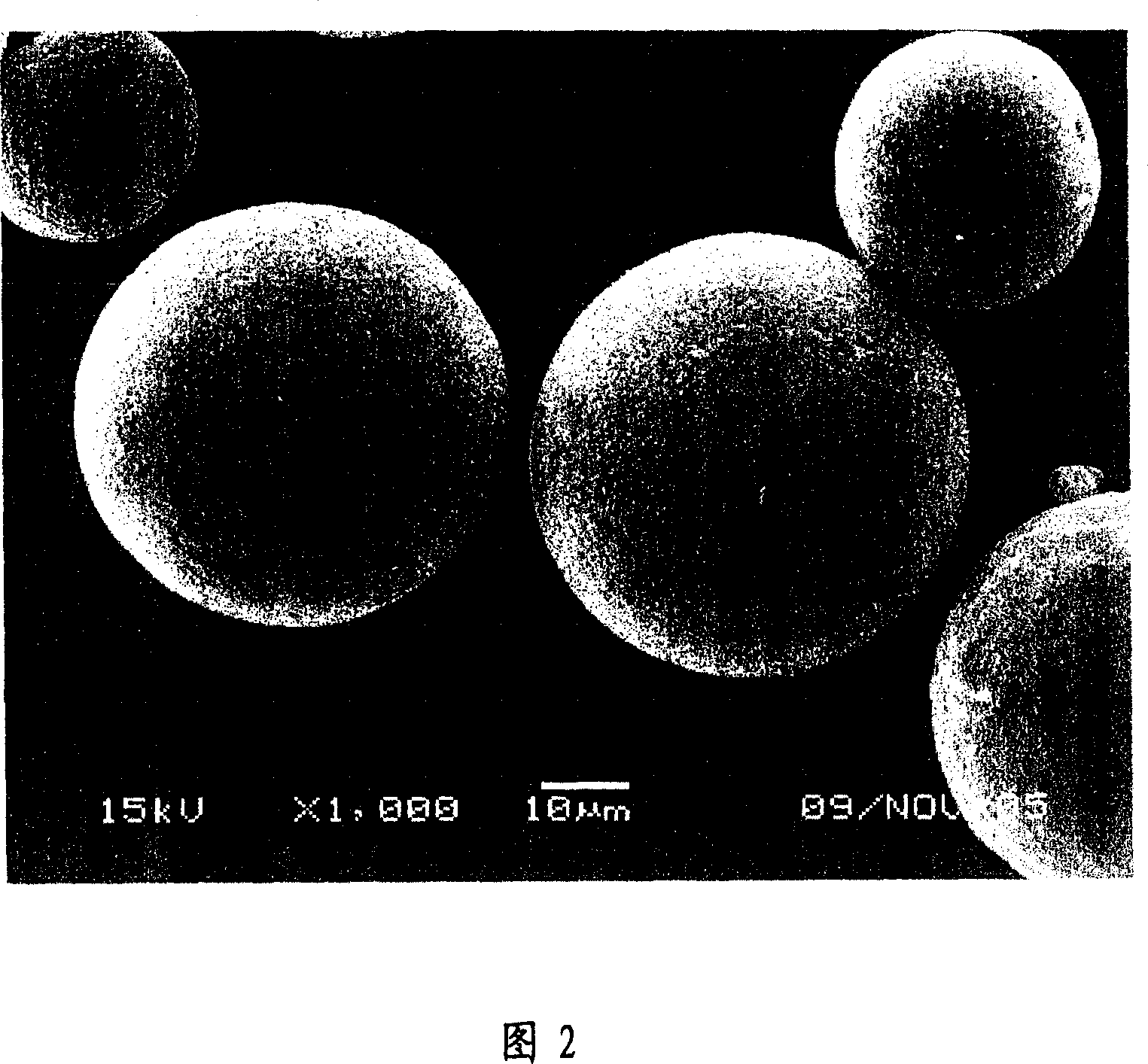
[0051] Dissolve 50mg of LXT-101 in 0.5ml of gelatin aqueous solution, 500mg of polylactide-glycolide (PLGA, lactide:glycolide=50:50, molecular weight 11000) in 5ml of dichloromethane, dichloromethane Those combined, at room temperature high-speed homogenizer per minute 10000 rpm high-speed stirring for 3 minutes to form a W / O emulsion; W / O emulsion is poured into 500ml containing 0.2% polyvinyl alcohol and 10% sodium chloride in the external water phase, Form W / O / W double emulsion, stir at a low speed of 500 rpm for 4 hours, volatilize the organic solvent, centrifuge, wash 3 times with distilled water, and freeze-dry for 20 hours to obtain a white powder with good fluidity. 76%, the particle size of the microspheres ranges from 5 to 80 μm, and the average particle size is 39.5 μm. The appearance is round and the particle size distribution is shown in Figure 1.
Embodiment 2
[0052] Example 2: Preparation of LHRH antagonist-LXT-101 microspheres by single milk method
[0053] Dissolve 10 mg of the main drug LXT-101 and 90 mg of PLGA (lactide:glycolide=75:25, molecular weight 36000) in a mixed solvent of dichloromethane and glacial acetic acid (7:3) (oil phase), Wherein the concentration of PLGA in the organic solvent is 300mg / ml, the above-mentioned oily phase (comprising principal agent and biodegradable polymer) is added to 2%PVA (polyethylene glycol) under the condition of stirring at a high speed of 7000 revolutions per minute for 5 minutes at room temperature. Vinyl alcohol) to form a W / O emulsion, and volatilize the organic solvent for 4 hours under low-speed stirring at 500 rpm. After the microspheres are solidified, centrifuge, wash, and freeze-dry to obtain the microspheres. The encapsulation rate of the microspheres prepared by this method can reach 78%, the measured particle size is 5-120 μm, and the average particle size is 48.9 μm. The ...
Embodiment 3
[0054] Example 3: Preparation of LHRH antagonist-LXT-101 microspheres by S / O / O method
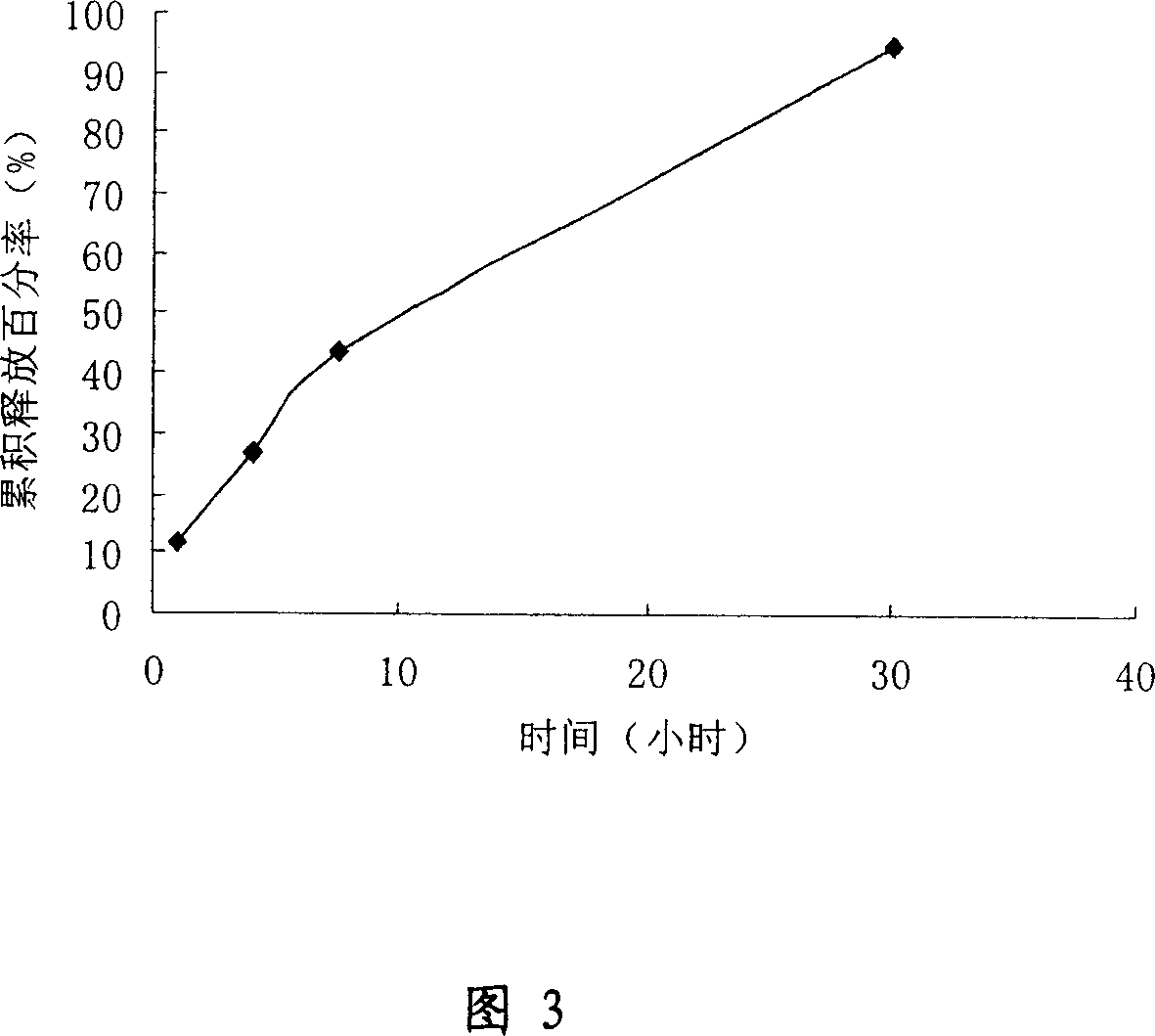
[0055] 75mg PLGA (lactide:glycolide=75:25, molecular weight is 20000) and 15mgPEG (molecular weight is 8000) (weight ratio 9:1) are dissolved in acetonitrile together, and concentration is C=500mg / ml, and micropowder The thawed LXT-101 powder (<20 μm) was suspended in the acetonitrile solution of the polymer, and dispersed uniformly under the condition of ice bath and 15000 rpm. The above solution was added dropwise to the continuous phase - cottonseed oil (containing 1.6% span 85) under the condition of mechanical stirring at 750rpm. After stirring for 5 h, petroleum ether (bp 50-110° C.) was added to cottonseed oil to extract residual acetonitrile. Stir for another 15 min, collect the microspheres by filtration, wash the microspheres with 250 ml petroleum ether and freeze-dry them for later use. The encapsulation rate of the microspheres obtained by this method can reach 65%, the measur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com