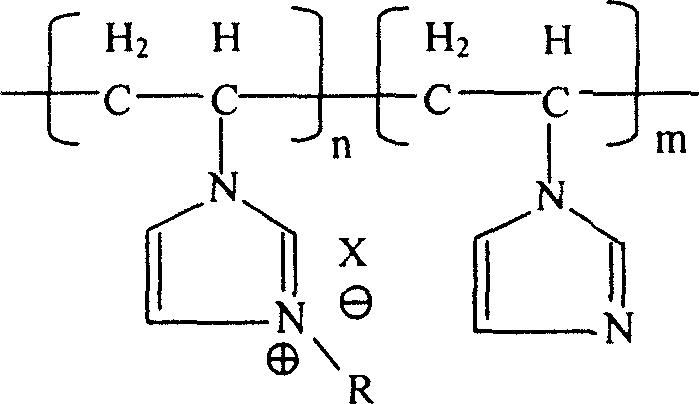
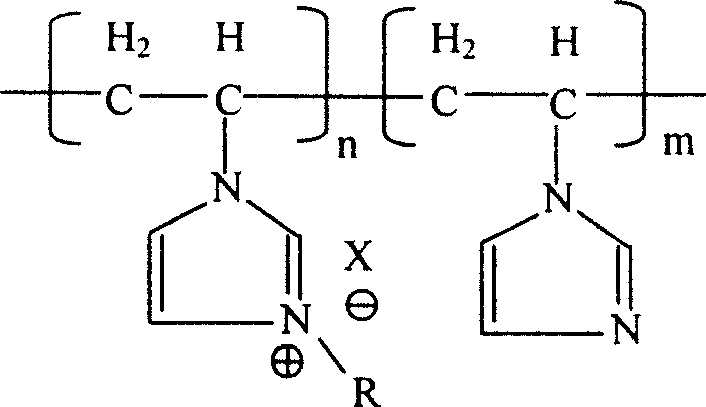
Poly(N- ethenyl-N'-alkyl- imidazole) ion liquid structural material and its preparation method
A technology for ionic liquids and structural materials, which is applied in the field of poly(N-vinyl-N'-alkyl-imidazole) ionic liquid structural materials and its preparation, and can solve problems that do not involve the molar fraction of structural segments containing ionic liquids , to achieve the effects of easy processing, wide electrochemical window and good mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 23.5 grams of vinylimidazole monomers (0.25 moles in total) were packed into a 150ml three-necked flask with a reflux condenser and a feeding tube, added benzene to make the solution 80ml, stirred, degassed, and filled with nitrogen for protection. After dissolving 0.1 g of azobisisobutyronitrile (AIBN) in 5 ml of benzene, place it in the feeding tube. The flask was heated to 60 °C, the AIBN solution was added into the flask from the feeding tube within one minute, and the polymerization reaction started. After 3 hours of polymerization reaction, the solvent was evaporated, and vacuum-dried in a vacuum oven at 80° C. for 24 hours to constant weight to obtain polyvinylimidazole. The weighing method is used for calculation and analysis, and the reaction conversion rate is 82.0% based on the addition of vinylimidazole monomer.
Embodiment 2
[0027] Put 4.7 grams (0.05 moles in terms of vinylimidazole monomer) of polyvinylimidazole into a three-necked flask with a reflux condenser, add 100 grams of absolute ethanol to dissolve it, and then add 8.2 grams (0.06 moles) of bromine Substitute n-butane (the molar ratio to vinylimidazole monomer is 1.2:1), heat up to ethanol reflux through a constant temperature bath under stirring, and the reaction continues for 24 hours, maintaining a weak nitrogen flow as a protection during the entire reaction process. gas. The ethanol solvent used in the reaction was evaporated, and the reaction product was vacuum-dried in a vacuum oven at 80°C for 24 hours to constant weight to obtain brominated n-butylpolyvinylimidazole, and the reaction combination rate was 85.0% based on vinylimidazole monomer .
Embodiment 3
[0029] Repeat Example 2, add 10.9 grams (0.1 mole) bromoethane (the molar ratio with vinylimidazole monomer is 2: 1) to replace brominated n-butane, and the reaction combination rate is calculated in terms of vinylimidazole monomer 89.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com