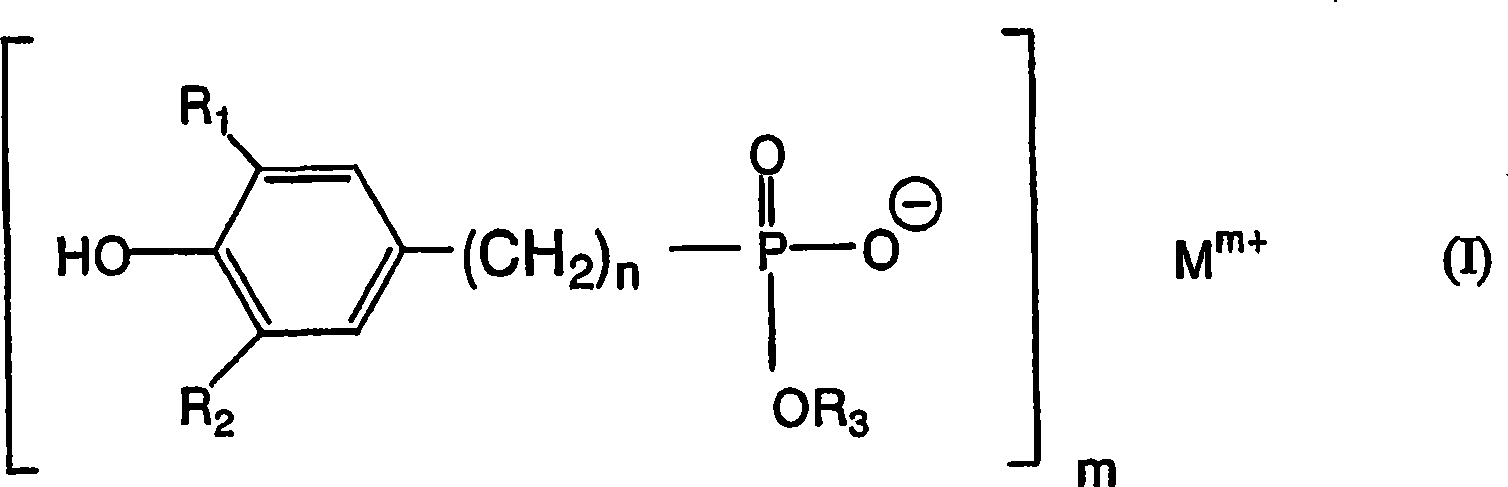
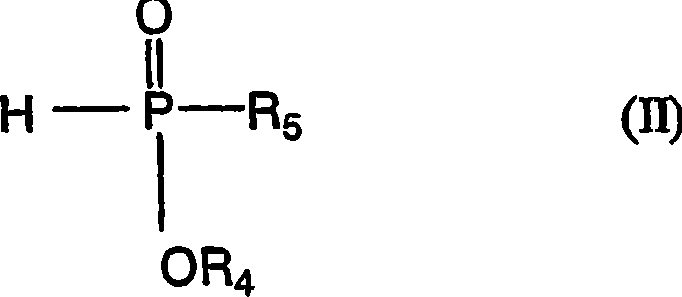
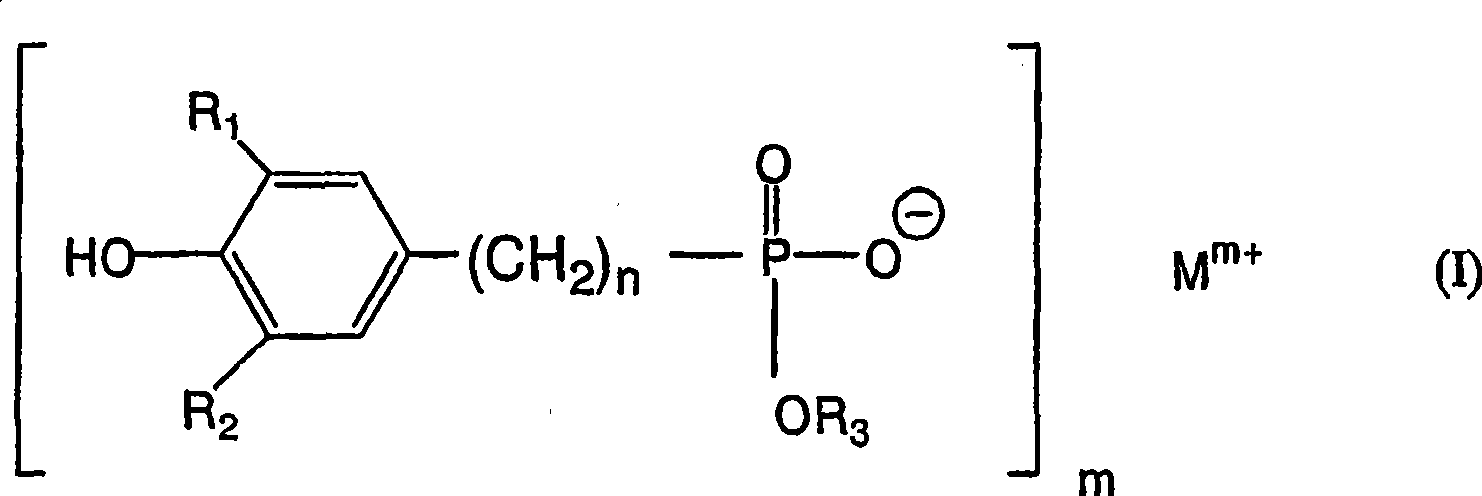
Polytrimethylene terephthalate
A technology of polytrimethylene terephthalate and polytrimethylene phthalate, which is applied in the field of polytrimethylene terephthalate and fibers containing the compound, and can solve problems such as inability to obtain fundamental improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] 100 parts by weight of dimethyl terephthalate and 70.5 parts by weight of 1,3-propanediol are mixed, and the mixture and 0.053 parts by weight of tetra-n-butyl titanate are charged into a device equipped with a stirrer, a rectifying tower and methanol to distill and condense in the reactor. The temperature of the reactor was gradually raised from 140° C., and the transesterification reaction was carried out while distilling off the methanol produced by the reaction to the outside of the system. Three hours after the start of the reaction, the internal temperature of the reactor reached 210°C.
[0115] Then, the resulting reaction product was transferred to a reaction vessel provided with a stirrer and a glycol distillation condenser, and the internal temperature of the reactor was slowly raised from 210°C to 265°C, while the pressure was dropped from normal pressure to 70Pa. High vacuum for polymerization. Monitor the melt viscosity in the reactor at any time, and sto...
Embodiment 2
[0122] In Example 1, without adding CDHMP, melt polymerization and solid state polymerization were performed to obtain a sheet with an intrinsic viscosity of 0.98 dL / g. Using a twin-screw extruder, this sheet was remelted at 260° C., and the content of CDHMP was adjusted to 0.1% by weight while adding it from a side feed hole. Then, it was cut using a strand cutter to obtain a sheet with an intrinsic viscosity of 0.93 dL / g. The obtained sheet was made into fibers in the same manner as in Example 1. The evaluation results are shown in Tables 1 and 2.
Embodiment 3
[0124] In Example 1, without adding CDHMP, melt polymerization and solid state polymerization were performed to obtain a sheet with an intrinsic viscosity of 0.93 dL / g. A 5% by weight methylene chloride solution of 21.2 parts by weight of CDHMP was uniformly poured into 100 parts by weight of the obtained sheet, and dried at normal temperature under a nitrogen stream. The obtained sheet was made into fibers in the same manner as in Example 1. The evaluation results are shown in Tables 1 and 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com