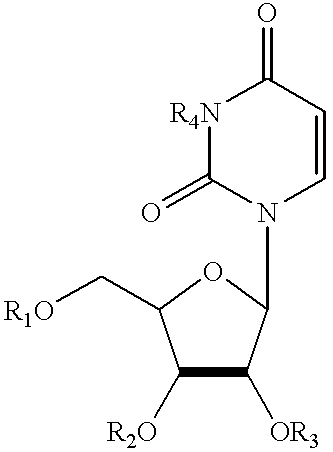
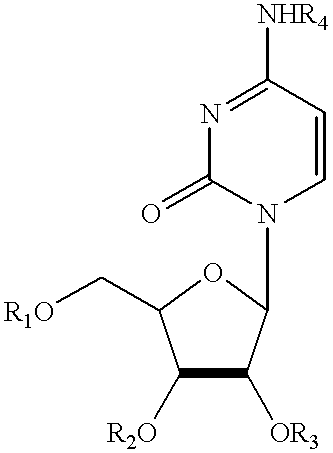
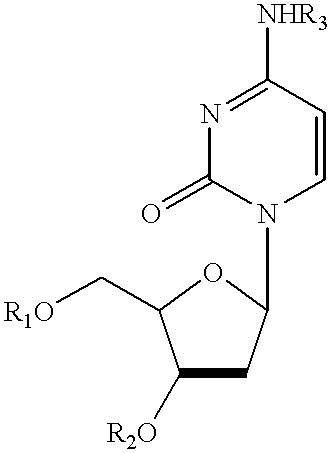
Treatment of chemotherapeutic agent and antiviral agent toxicity with acylated pyrimidine nucleosides
a technology of acylated pyrimidine and chemotherapeutic agents, which is applied in the direction of biocide, antinoxious agents, drug compositions, etc., can solve the problems of reducing affecting the production of granulocytes, and inhibiting the production of new blood cells, so as to achieve effective prevention or treatment of toxic symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0224] Oral Administration of Triacetyluridine Ameliorates Hematologic Toxicity of 5-fluorouracil
[0225] Purpose
[0226] This study was undertaken in order to determine if oral administration of TAU could rescue mice from 5-FU toxicity more effectively than oral administration of uridine itself. Bone marrow cellularity and peripheral blood cell counts were used as an index of 5-FU toxicity.
[0227] Methods
[0228] Forty-five female Balb / C mice (20 grams each) were given 5-fluorouracil (150 mg / kg, i.p.) at 12:00 noon on the initial day of the experiment. These animals were then divided into 5 groups: control (water, p.o.), oral uridine at 400 mg / kg / dose, oral uridine at 800 mg / kg / dose, parenteral (i.p.) uridine at 400 mg / kg / dose, and oral TAU at 500 mg / kg / dose.
[0229] Two hours after administration of 5-FU, the rescue treatments with uridine or triacetyluridine were begun. Groups received their designated treatment at 2:00 p.m., 4:00 p.m., and 6:00 p.m. on the day of 5-FU administration, and...
example 2
[0244] TAU Accelerates Hematopoietic Recovery in 5-FU-treated Animals in a Dose Dependent Manner
[0245] Purpose
[0246] The purpose of this experiment was to confirm and extend the previous findings that orally administered TAU accelerates hematopoietic recovery in mice treated with 5-fluoruracil (5-FU), and to observe the relationship between increasing doses of TAU and the responses of the hematopoietic system of these 5-FU-treated mice.
[0247] Methods
[0248] Seventy female Balb / C mice weighing approximately twenty grams were each given an i.p. injection of 5-FU (150 mg / kg) at 1:00 p.m. on the initial day of the study. These animals were then divided into five different treatment groups: control (water), and oral TAU at doses of 100, 250, 500, and 1,000 mg / kg / treatment. The test compounds were then given at 3:00, 5:00, 7:30, and 10:00 p.m. on the day of 5-FU administration; at 9:00 a.m., 11:00 a.m., and 1:00, 3:00, 6:00, and 10:00 p.m. the following day; and a final administration at 1...
example 3
[0264] Acyl Derivatives of Uridine Ameliorate Bone Marrow Toxicity of 5-fluorouracil
[0265] Purpose
[0266] The purpose of this experiment was to test and compare the efficacy of uridine and derivatives of uridine in attenuating damage to the hematopoietic system of mice caused by the chemotherapeutic agent 5-flourouracil (5-FU).
[0267] Methods
[0268] Ninety-eight female Balb / C mice weighing approximately 20 grams each were given a one-time 150 mg / kg injection (i.p.) of 5-FU at 1 p.m. on the initial day of the study. These animals were then divided into seven groups: control (saline), uridine (300 mg / kg / treatment), triacetyluridine (TAU; 455 mg / kg / treatment), benzoyluridine (BU; 428 mg / kg / treatment), ethoxycarbonyl (ECU; 389 mg / kg / treatment), octanoyluridine (OU; 455 mg / kg / treatment), and valeryluridine (VU; 403 mg / kg / treatment). All of these doses are equimolar, and were administered in a volume of 0.4 ml by i.p. injection. The groups were treated at 3:30, 6:00, and 8:30 p.m. on the ini...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com