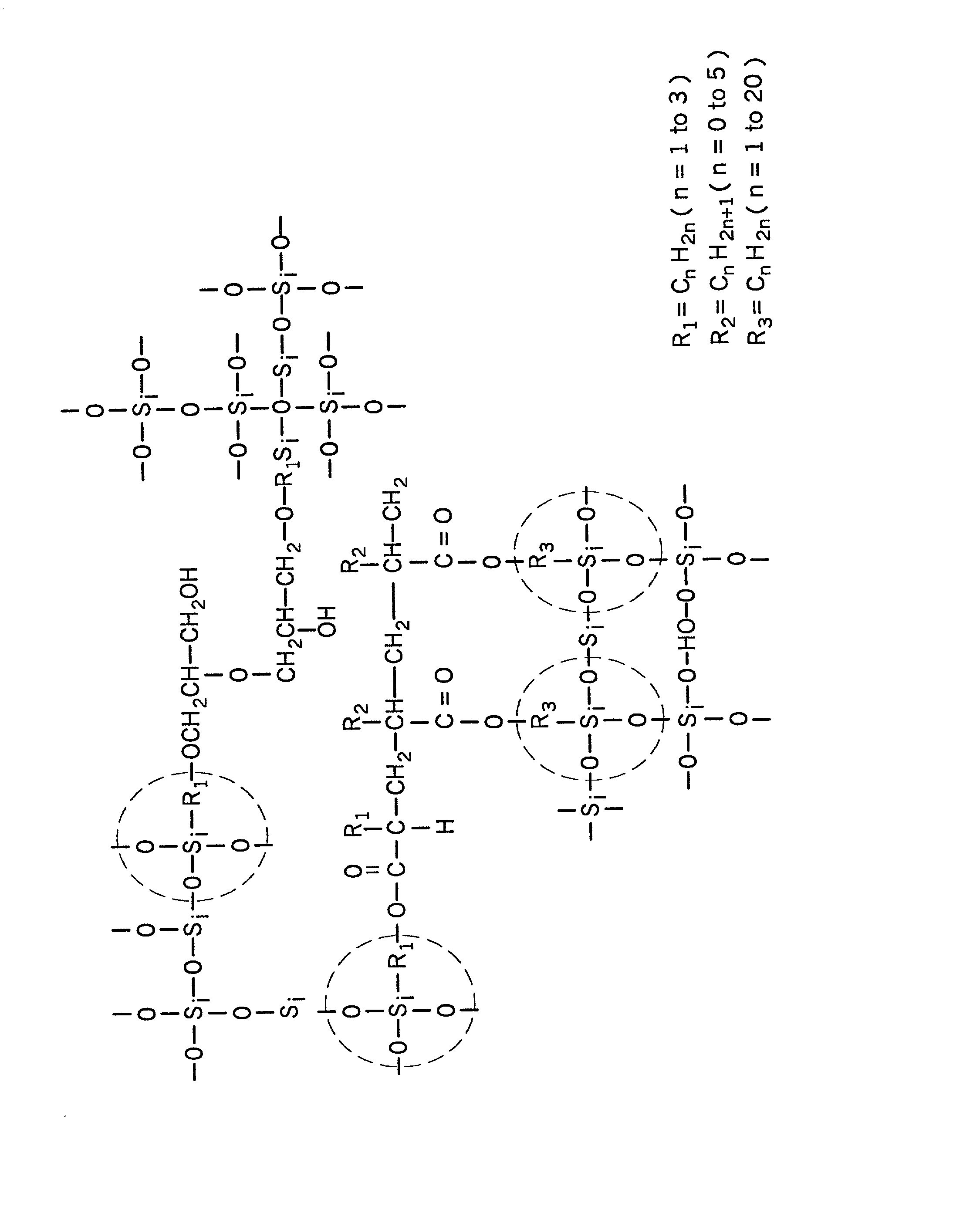
Organic-inorganic hybrid polymer and method of making same
a hybrid polymer and organic polymer technology, applied in the direction of synthetic resin layered products, coatings, transportation and packaging, etc., can solve the problems of reduced optical clarity, difficult to achieve good bonding, and inability to use lenses made of organic polymers, so as to achieve the effect of fast cure on the surface of plasti
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
[0069] A coating solution is prepared by mixing 104.0 g of tetraethyl orthosilicate, 45.0 g of glycidoxypropyltrimethoxysilane, 5.0 g methacryloxypropyltrimethoxysilane, 119.0 g of isopropyl alcohol, 43.0 g of water, 0.4 g of 2M HCl and 3.2 g of 2M acetic acid. The solution is stirred at room temperature to partially hydrolyze the silane groups until a clear solution is obtained. The solution is then heated up to and maintained at 60-70.degree. C. for 1-2 hours while continuing the stirring to completely hydrolyze all silane groups. The solution is then cooled to room temperature, followed by the addition of 1.6 g of Brij.RTM.98 surfactant and 1.2 g of aluminum acetylacetonate catalyst. The solution then is stirred to dissolve the solids, and to obtain a homogeneous and clear solution.
[0070] A CR-39 lens is cleaned dried and immersed into the coating solution and withdrawn edgewise with the lens surfaces generally perpendicular to the solution surface at a rate of 5-15 cm / min. In th...
example ii
[0074] A scratch resist coating solution is prepared by mixing 313 g of tetraethyl orthosilicate, 200 g of glycidoxypropyltrimethoxysilane, 40 g methacryloxypropyltrimethoxysilane, 472 g of isopropyl alcohol, 144 g of water, 1.2 g of 2M HCl and 10.8 g 2M HAc. The solution is stirred at room temperature to partially hydrolyze the silane groups until a clear solution is obtained. The solution is then heated up to and maintained at 60-70.degree. C. for 1-2 hours while continuing the stirring to completely hydrolyze all silane groups. The solution is then cooled to room temperature followed by the addition of 6.0 g of Brij.RTM.98 surfactant and 4.0 g of aluminum acetylacetonate catalyst. The solution is then stirred to dissolve the solids, and obtain a homogeneous and clear solution.
[0075] A CR-39 lens is cleaned, dried and immersed into the coating solution followed by withdrawal edgewise with the lens surfaces generally perpendicular to the solution surface at a rate of 5-15 cm / min. T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com