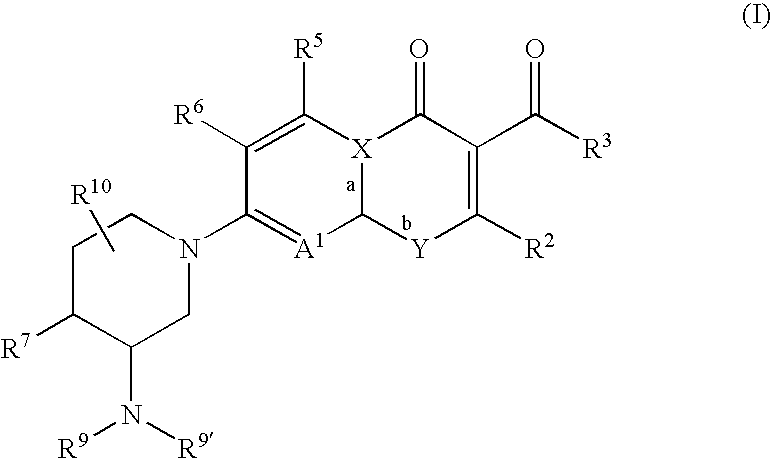
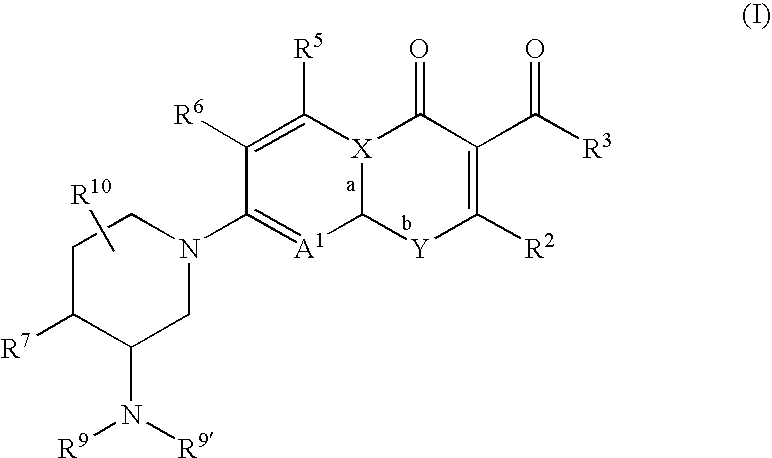
Antimicrobial quinolones, their compositions and uses
a technology of quinolones and quinolones, applied in the field of antimicrobial compounds, can solve the problems of limited capacity of existing antibacterials to overcome the threat, few antimicrobials are produced that are truly clinically acceptable, and a significant threat to public health in the developed world, so as to reduce the susceptibility to microbial resistance, improve pharmacology, and reduce the effect of toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example a
PRECURSOR EXAMPLE A
[0142] 182
3-methoxy-2,4,5-trifluorobenzoyl chloride
[0143] 3-Methoxy-2,4,5-difluorobenzoic acid (43.9 g) is suspended in dichloromethane (30 mL) and oxalyl chloride (25 mL) is added followed by 4 drops of dry dimethyl formamide (DMF). The mixture is stirred at room temperature for 6 hours and the solvent is removed by evaporation to afford the desired product.
Ethyl 2,4,5-trifluoro-3-methoxy-benzoyl acetate
[0144] Monoethyl malonate (26.4 g) is dissolved in tetrahydrofuran (THF) (700 mL). The solution is cooled at -50.degree. C. and n-butyllithium (160 mL 2.5 M) is added, keeping the temperature below -50.degree. C. The temperature is initially raised to 0.degree. C. and cooled back to -50.degree. C. 3-methoxy-2,4,5trifluorobenzoyl chloride (20.6 g) is added, keeping the temperature at -50.degree. C., then the reaction mixture is warmed to room temperature. Hydrochloric acid is added until the pH becomes acidic. The organic phase is washed with sodium bicarbonate and...
example 1
7-(Trans-3-amino-4-ethyl-piperidine-1-yl)-1-Cyclopropyl-1,4-dihydro-6-fluo- ro-8-methoxy-4-oxo-quinoline-3-carboxylic acid hydrochloride
[0202] 189
[0203] 1-Cyclopropyl-1,4-dihydro-6,7-difluoro-8-methoxy-4-oxo-quinoline-3-- carboxylic acid (Precursor A) (0.059 g), Trans,3-tert-butoxycarbonylamino-- 4-ethyl-piperidine (Precursor F) (0.048 mg) and triethylamine (0.075 mL) are dissolved in N-methyl-pyrrolidone (2 mL). The reaction mixture is stirred at 80.degree. C. for 5 hours, then is poured on an ice / water mixture. The pH is lowered to 2 with diluted HCl and the resulting precipitate is filtered. The solid is then suspended in ethanol and 6N HCl is added. After 18 hours at room temperature, the desired final product is collected by filtration.
example 2
7-[Trans-3-amino-4-(2-hydroxy-ethyl)-piperidine-1-yl]-1-Cyclopropyl-1,4-di- hydro-6-fluoro-8-methoxy-4-oxo-quinoline-3-carboxylic acid hydrochloride
[0204] 190
[0205] A procedure similar to Example 1 above is used, using 1-Cyclopropyl-1,4-dihydro-6,7-difluoro-8-methoxy-4-oxo-quinoline-3-carbox- ylic acid (Precursor A) and Trans 4-(2-hydroxy-ethyl)-3tertbutoxycarbonyla- mino-piperidine (Precursor G) as the starting materials. The procedure utilizes the same reaction conditions and molar ratio of reactants as Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com