Chondroitin synthase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
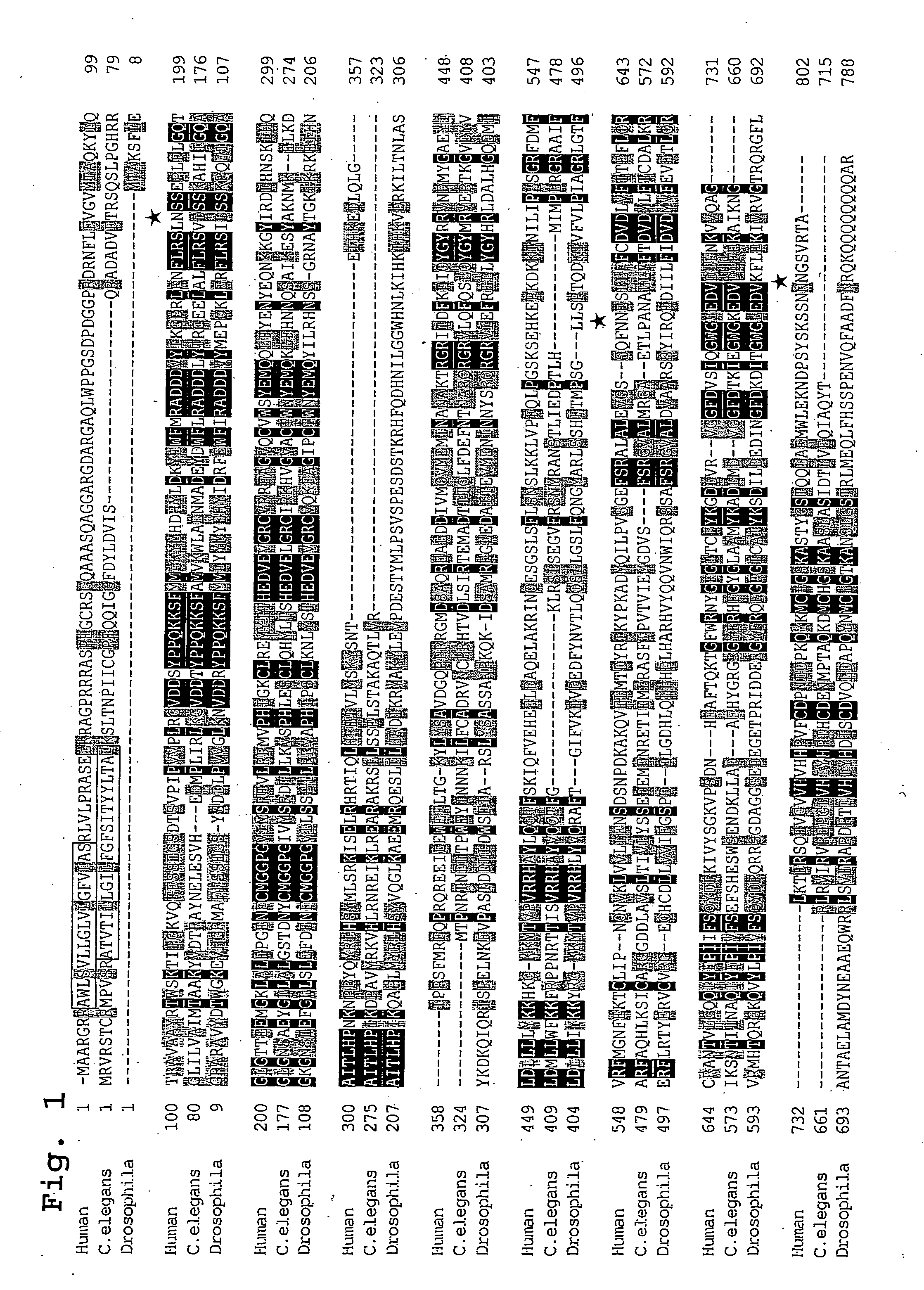
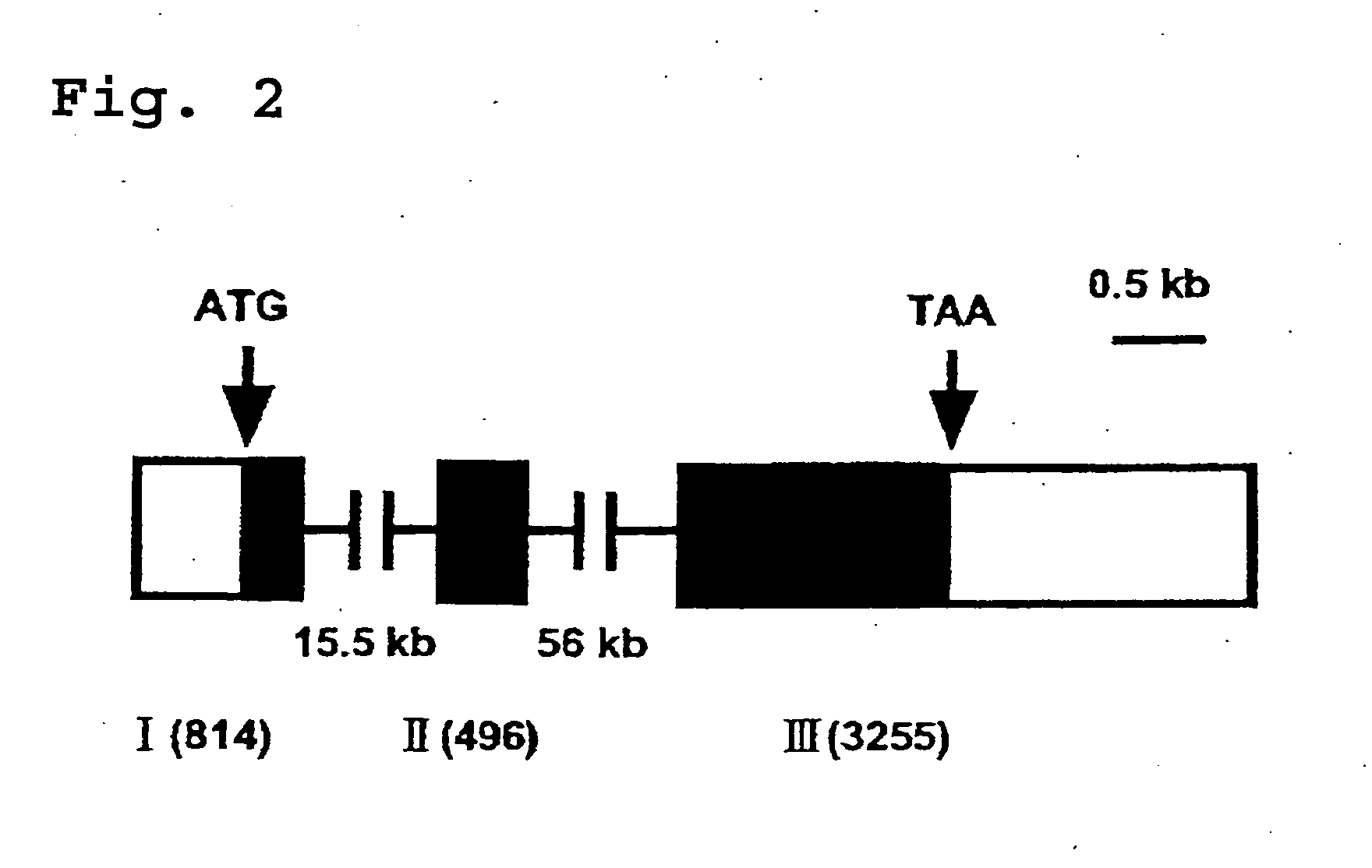
[0153] (1) In Silico Cloning of Novel Human Glycosyltransferase cDNA
[0154] Screening of HUGE protein database at Kazusa DNA Research Institute (in Chiba Prefecture; http: / / www.kazusa.or.jp / huge / ) was conducted by the keywords "one transmembrane domain" and "galactosyltransferase family". As a result of this, one clone (KIAA0990; GenBank.TM. accession number AB023207) was identified. An analysis of a nucleotide sequence of this clone revealed that this clone includes (i) a 5'-untranslated region of 494 bp, (ii) a single open reading frame of 2406 bp coding for a protein of 802 amino acids with three potential N-glycosylation sites (marked with asterisks in FIG. 1), and (iii) a 31-untranslated region of about 1.7 kb with a presumptive polyadenylation signal. The nucleotide sequence and an amino acid sequence deduced from the same are shown in SEQ. ID. NO: 1, whereas only the amino acid sequence is shown in SEQ. ID. NO: 2.
[0155] The clone was acquired from Kazusa DNA Research Institute...
example 2
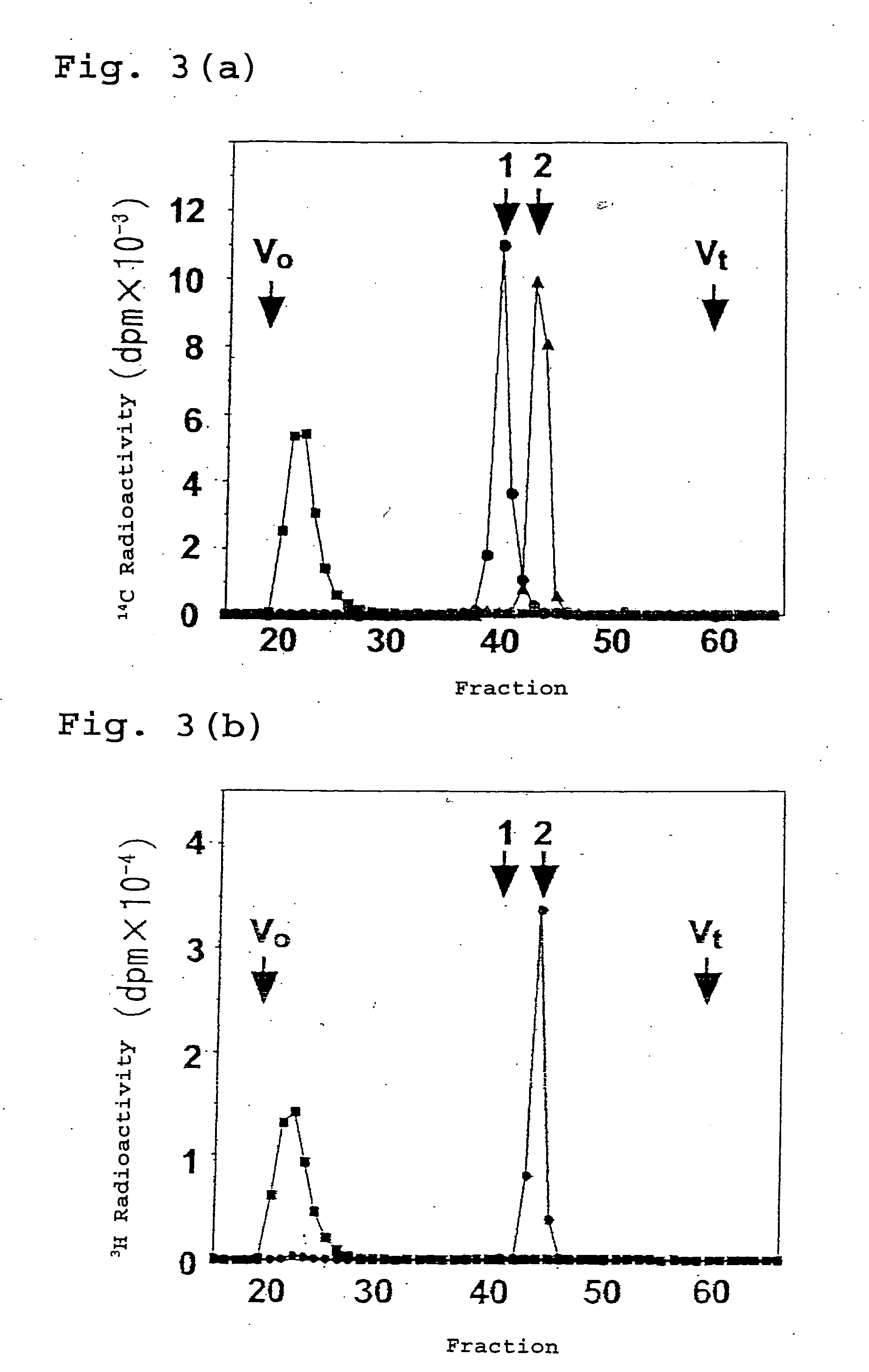
[0175] A commercially-available human 12-lane multiple tissue Northern blot (Clontech) membrane was used for analysis. To each lane, 1 .mu.g of a polyadenylated RNA was applied. The membrane was probed with a gel-purified and radiolabeled (>1.times.10.sup.9 cpm / .mu.g) 0.84 kb chondroitin-synthase-specific fragment corresponding to nucleotides 631-1469 of the KIAA0990 cDNA (GenBank.TM. accession number AB023207).
[0176] As a result, a single band of up to 5.0 kb was demonstrated for all human tissues, at least in this analysis (FIG. 4). The degree of the expression of the chondroitin synthase gene which is prevalent in human tissues varied with the types of human tissues. Notably, a particularly strong expression of the mRNA was observed in the placenta. The expressions observed in the spleen, lung, and peripheral blood leukocytes were also strong but not as much as that of the placenta. This result corresponds to an observation that chondroitin sulfate proteoglycans are distributed a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility (mass) | aaaaa | aaaaa |
| Catalytic activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com