Method of preventing or reducing scarring of human skin
a scarring and human skin technology, applied in the field of human skin scarring prevention or scarring reduction, can solve the problems of reducing the individual's vision, reducing the severity of so-called "normal" scarring, and still varying dramatically between individuals, so as to prevent or reduce the formation of scar tissue, prevent or reduce scarring, and improve the speed of re-epithelialisation of wounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
In Vitro Study
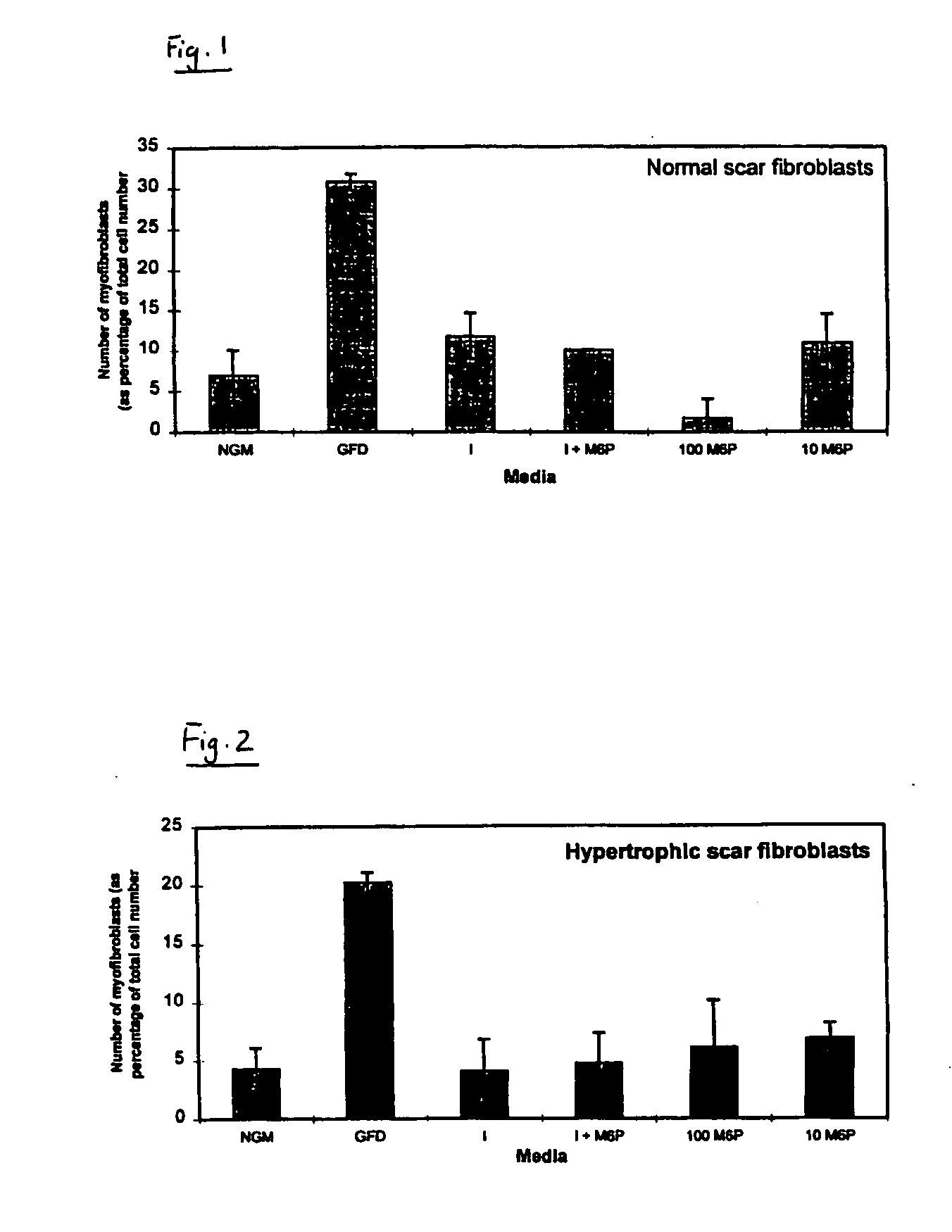
[0059] One of the most important cell types in both normal and pathological scar formation is the myofibroblast. These cells, which differentiate from the unwounded tissue cell type (fibroblasts), are responsible for laying down scar tissue. Indeed myofibroblasts remain present in hypertrophic scars up to four years after the original wounding event. An in vitro assay was accordingly developed to identify actives which prevent or reduce myofibroblast formation and thus identify actives which are effective in reducing and / or preventing scar tissue formation.
[0060] Fibroblast cultures were initiated from normal skin, normal scars, hypertrophic scars (HTS) and burns scars.
[0061] Each culture was split into seven parts (A to G) and grown in the following different growth media:
[0062] (A) was grown in normal growth medium (hereinafter referred to as NGM) which consisted of Dulbecco's modified Eagles Medium (DMEM) plus 10% Foetal Calf Serum (FCS);
[0063] (B) was gr...
example 2
In Vivo Study
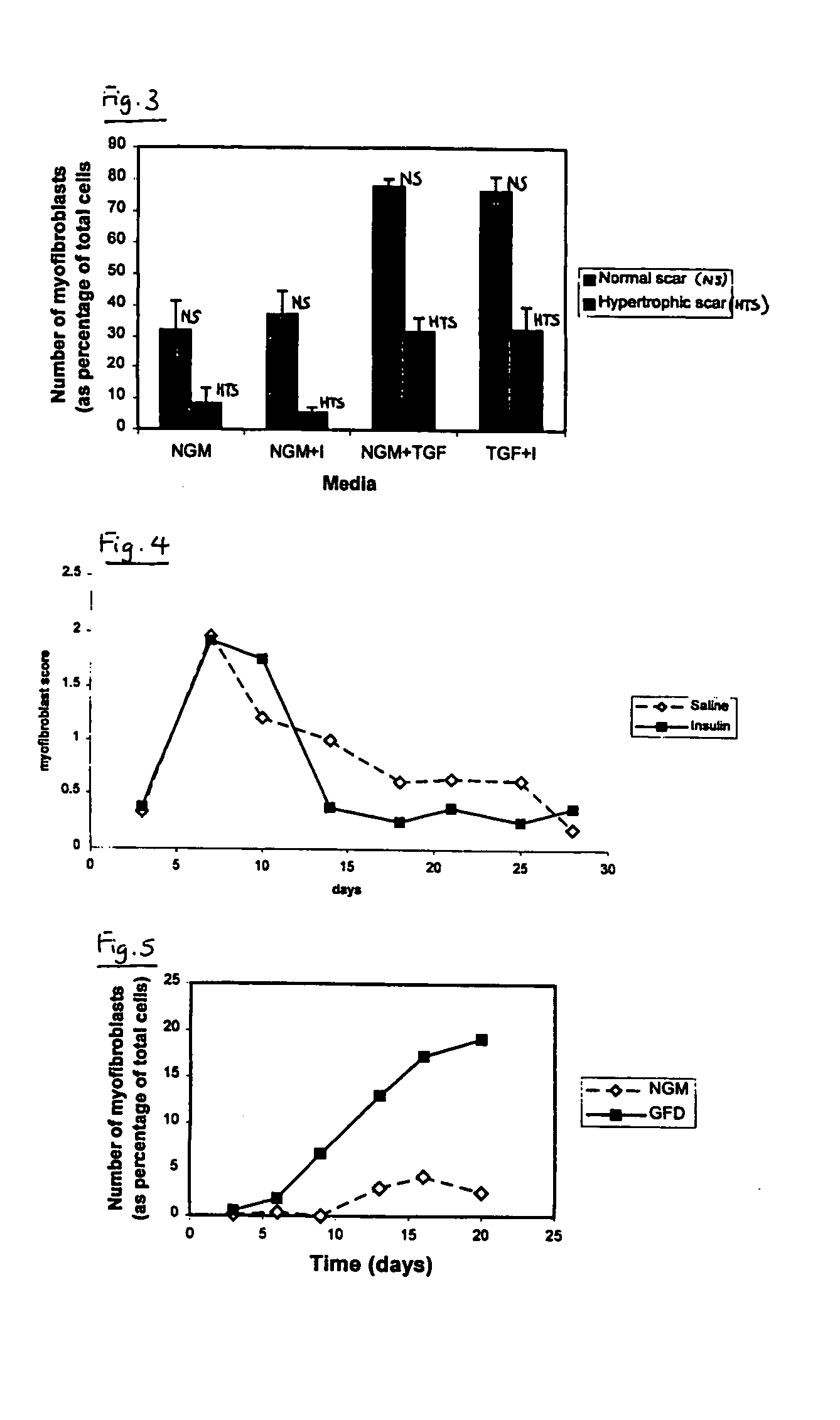
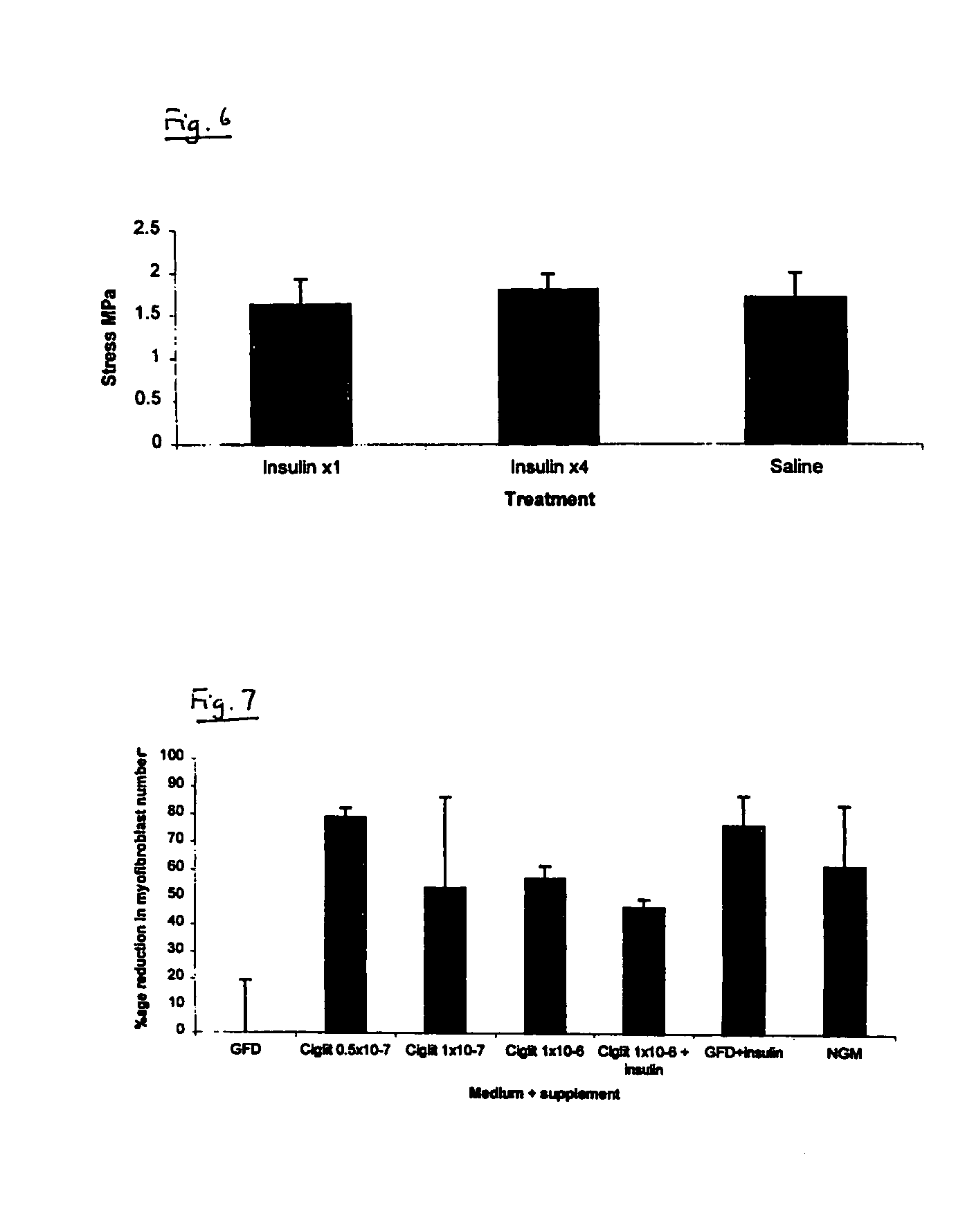
[0075] The efficacy of an exemplifying treatment protocol using insulin in vivo was determined using a murine incisional wound healing model in the manner described below.
[0076] After general anaesthesia, both posterior flanks on male BALB / c mice were shaved and cleaned with chlorhexidine in spirit. Two 1.5 cm wounds were marked (one in each flank) parallel to the spine with permanent marker. Full thickness incisions were made along these marks down to the level of the chest wall. Both lateral skin flaps were dissected from the underlying chest wall and the wound assessed from haemorrhage. The wound edges were then infiltrated with test substance or vehicle only control, as appropriate, by injection into the wound edges. Insulatard® was diluted in normal saline (0.9%) to give a final test solution of 1 IU / ml. 0.15 ml of this test solution (0.15 IU) was applied to one of the test wounds and saline alone was applied to the contralateral wound on the same animal. Wounds ...
example 3
[0079] The following injectable composition was prepared in conventional manner. It was found both to prevent and reduce scar tissue formation when locally injected into wounds in accordance with the present invention. The formulation is also suitable for topical application.
[0080] Each millilitre of the formulation contains:
[0081] 100IU of insulin (human insulin (pyr) which is of recombinant origin produced in yeast), 3.78 mg dibasic sodium phosphate, 1.76 mg m-cresol, 0.715 mg phenol, zinc oxide, (content adjusted to provide 0.025 mg zinc ion), 0.28 mg protamine sulphate, 16 mg glycerin, and water to dilute to the required concentrations. The pH range of the composition is preferably 7.0-7.8, but 10% sodium hydroxide or hydrochloric acid may be used to adjust the pH, as required.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| breaking strength | aaaaa | aaaaa |
| roughness/smoothness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com