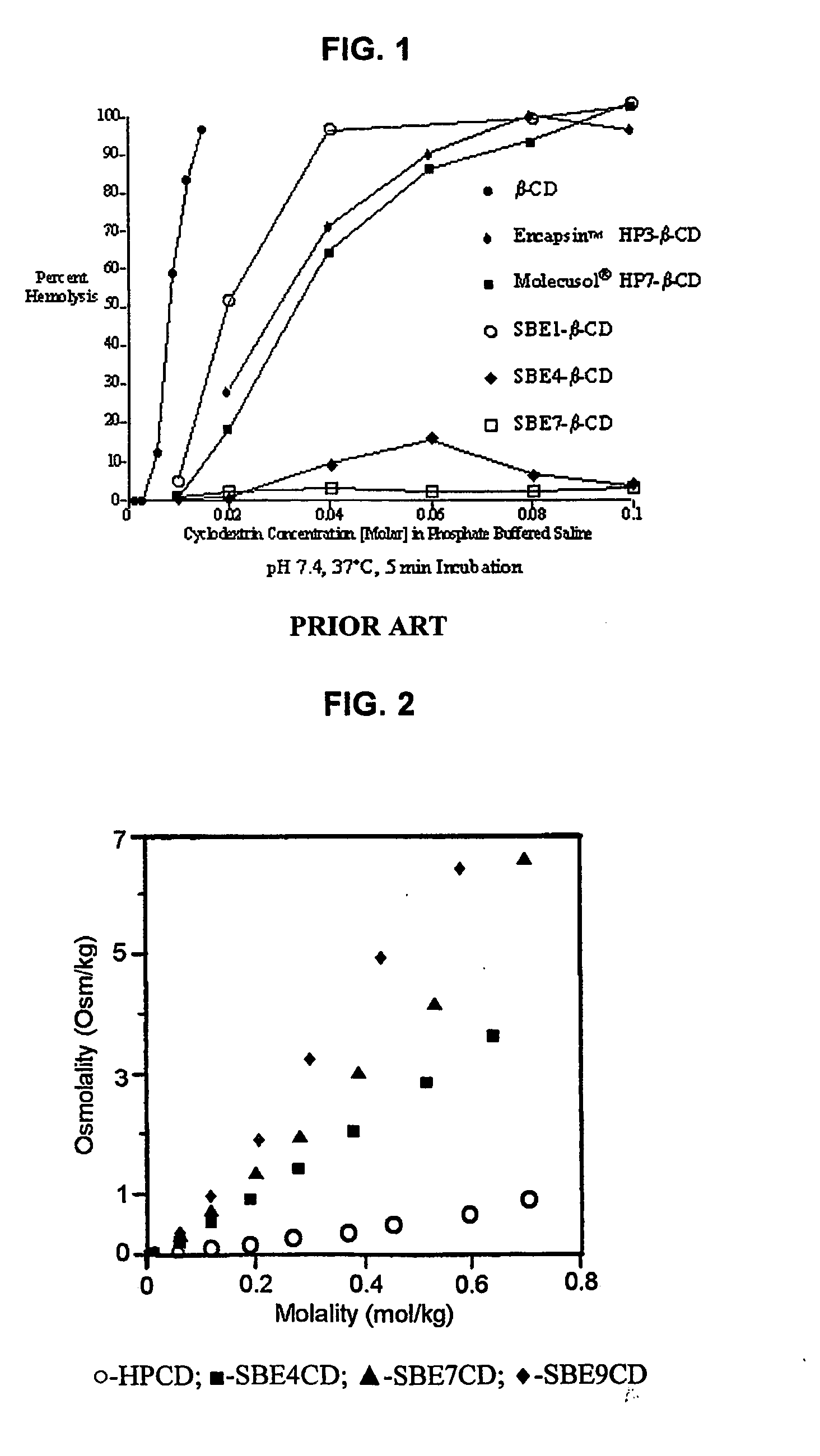
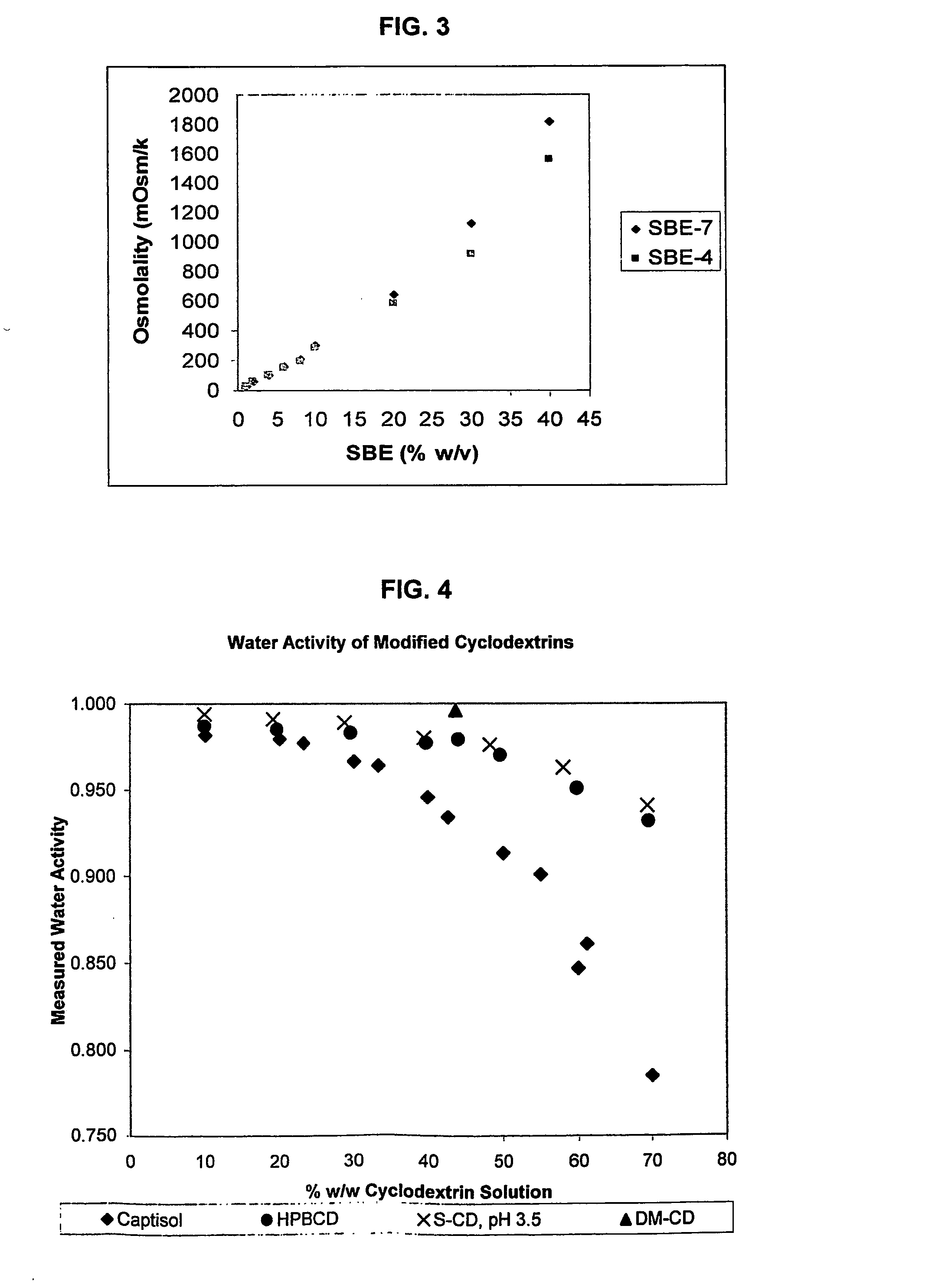
Use of sulfoalkyl ether cyclodextrin as a preservative
a technology of sulfoalkyl ether and cyclodextrin, which is applied in the field of use of sulfoalkyl ether cyclodextrin as a preservative, can solve the problems of reducing the ability of bacterial growth to continue, especially in the direction of application, biocide, microorganisms, etc., and achieves enhanced solubility, enhanced chemical, thermochemical, hydrolytic and/or photochemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0147] Exemplary formulations according to the invention were made according to the following general procedure. CAPTISOL® cyclodextrin was dissolved in water to form a solution containing about 220 mg / mL of CAPTISOL® cyclodextrin. Propofol was added to the SAE-CD containing solution until a concentration of about 10 mg / mL propofol was reached. An additional preservative was added and the pH adjusted with sodium hydroxide / hydrochloric acid as indicated in the table below.
AmountIngredientFormulation 1Formulation 2Formulation 3Propofol 10 mg / mL 10 mg / mL 10 mg / mLCAPTISOL ®220 mg / mL220 mg / mL220 mg / mLcyclodextrinEDTA—0.05 mg / mL —Sodium Metabisulfite——0.25 mg / mL pHno adjustment8.25.5Sterile Water forto volumeto volumeto volumeInjection
example 2
[0148] The following example describes an exemplary method for the preparation of a preserved formulation according to the invention as a solid for reconstitution.
IngredientAmountPropofol 20 mg / mLCAPTISOL ® cyclodextrin432 mg / mLWaterto volume
[0149] CAPTISOL® cyclodextrin was dissolved in water to form a solution containing about 0.2 Molar (approximately 432 mg / mL) of CAPTISOL® cyclodextrin. Propofol was then added to the SAE-CD containing solution with stirring until a concentration of about 20 mg / mL propofol was reached. The solution was lyophilized to generate a solid formulation. Prior to use as a solution, sufficient sterile water for injection is added to the solid formulation to generate a final solution containing propofol 10 mg / mL.
example 3
[0150] The growth retarding capability of three formulations of the invention, formulations 1, 2, and 3 from example 1, were compared to two marketed formulations of the active agent that do not contain a cyclodextrin of the invention. The two marketed formulations, Diprivan Injectable Emulsion 1% and Propofol Injectable Emulsion 1% each contain 10 mg / mL propofol, 100 mg / mL soybean oil, 22.5 mg / ni glycerol, and 12 mg / mL egg lecithin. In addition, Diprivan Injectable Emulsion 1% contains 0.05 mg / mL disodium edetate (EDTA) at a pH of 7.0 to 8.5, and Propofol Injectable Emulsion 1% contains 0.25 mg / mL sodium metabisulfite at a pH of 4.5 to 6.4. The antimicrobial preserving capability of the formulations was evaluated in duplicate employing a liquid to liquid matrix against seven test organisms, at three exposure intervals, and at two exposure temperatures, then quantitated using membrane filtration. Approximately 50-200 colony formation units (CFU) per mL of five standard organisms rec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Pore size | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com