Nucleic acid probes and methods to detect and/or quantify nucleic acid analytes
a nucleic acid and probe technology, applied in the field ofmolecular biology, can solve the problems of increasing the fluorescence of the detector dye, contaminating and false positives, and affecting the efficiency of the hydrolysis probe in homogeneous assays, and achieves enhanced binding affinities and low fluorescent effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
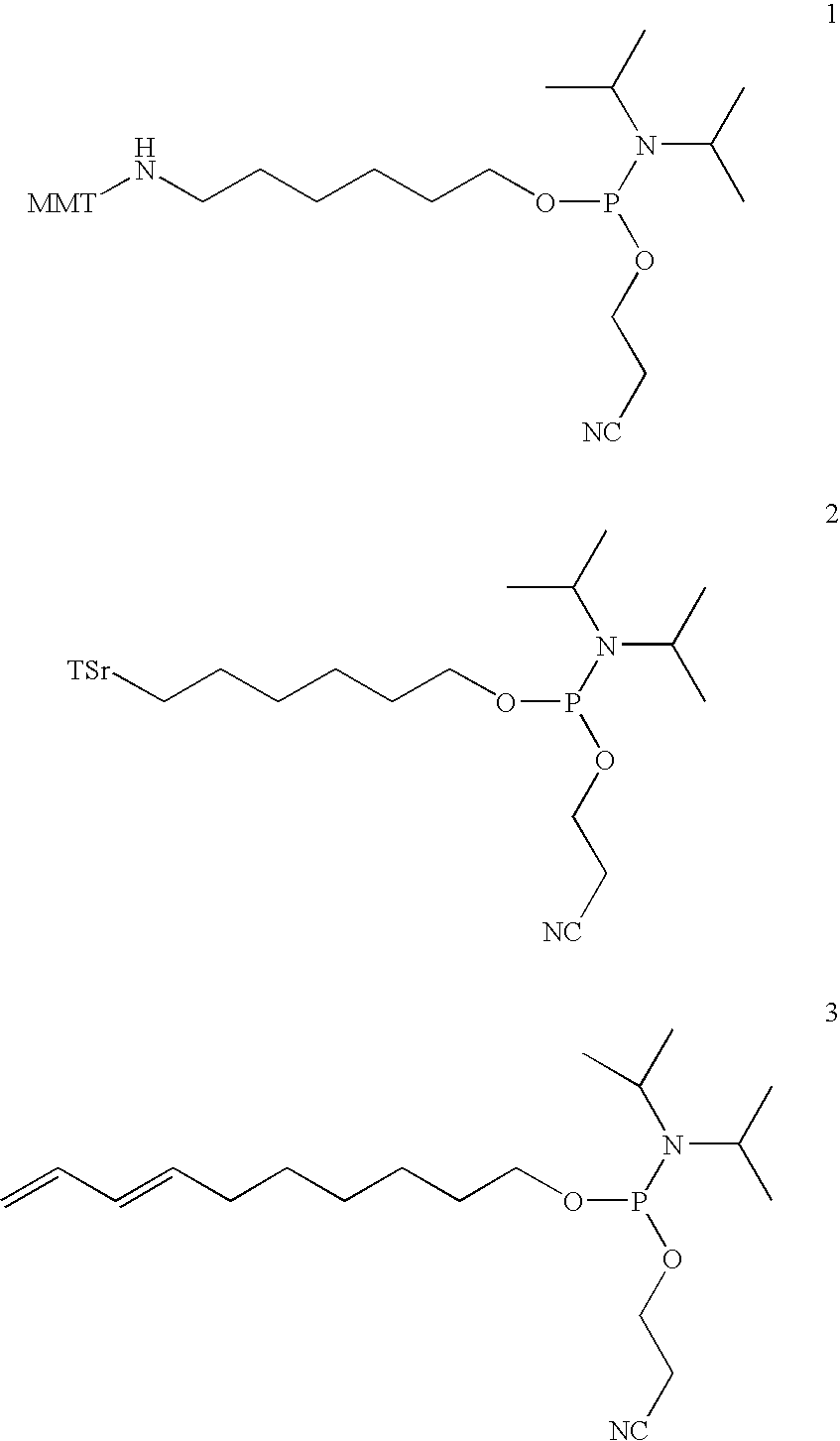
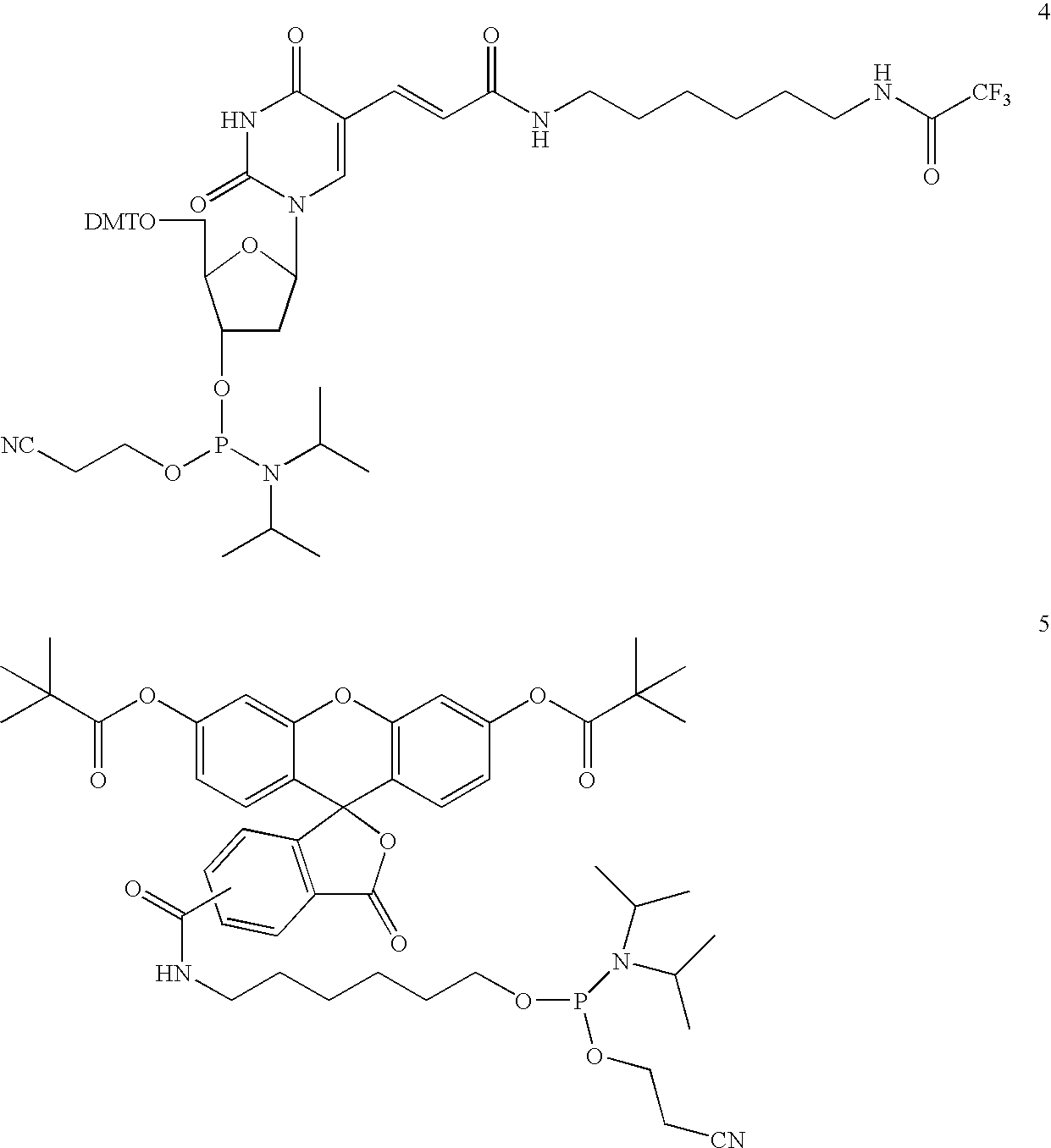
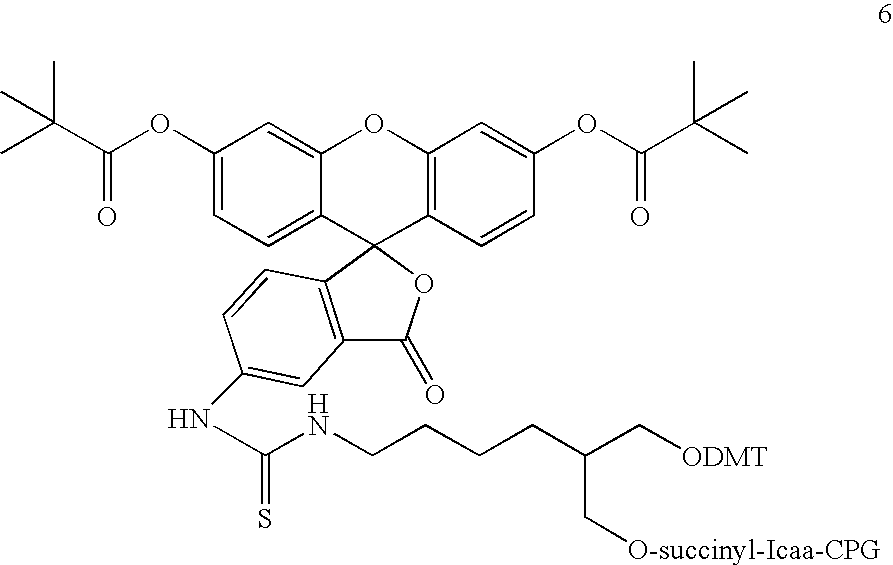
Synthesis of bifunctional linker (13): 1-(N-4-(9-fluorenylmethoxycarbonyl) aminobutyryl)-amino-3-(N-4-(4-methoxytrityl)-aminobutyryl)-aminopropan-2-ol cyanoethoxy-diisopropylaminophosphoramidite
[0197] The synthesis of bifunctional linker 13 is outlined in Scheme 1.
N4-(9-fluorenylmethoxycarbonyl)aminobutyric acid. 4-Aminobutyric acid (12.4 g, 120 mmol) was dissolved in a solution of Na2CO3 (50.9 g, 480 mmol) in water (450 mL). To this mixture a solution of 9-fluorenylmethyl chloroformate (Fmoc-chloride, 25.8 g, 100 mmol) in 1,4-dioxane (60 mL) was added under vigorous stirring at ambient temperature. The initially formed precipitate disappeared during the reaction (approx. 3 hours). After the Fmoc-chloride was completely consumed (TLC:CH2Cl2 / EtOH, 90:10; Rf (product) 0.67), the mixture was carefully adjusted to pH 2 by adding concentrated HCl. The slurry obtained was overlaid with ethyl acetate (˜2.5 L), mixed and the two phases were allowed to separate. The organic phase was was...
example 2
Synthesis of the bifunctional linker (14): 1-(N-6-(9-fluorenylmethoxycarbonyl)aminocaproyl)-amino-3-(N-6-(cyclohexa-2,4-dienylmethoxycarbonyl)aminocaproyl)-aminopropan-2-ol cyanoethoxydiisopropylaminophosphoramidite
[0206] The synthesis of bifunctional linker 14 is outlined in Scheme 2.
Ethyl-ε-aminocaproate hydrotosylate. A mixture of ε-aminocaproic acid (70.0 g, 533.8 mmol), toluenesulfonic acid (111.7 g, 1.1 eq.) and ethanol (1.6 L) was heated to reflux for 20 hours. Thereafter, TLC (CH2Cl2 / EtOH / TEA, 85:10:5) revealed complete conversion. The reaction mixture was concentrated until solids started to precipitate. The precipitation was driven to completion by adding diethyl ether (1.5 L). The white solid was filtered, washed with diethyl ether, and dried in vacuo affording 174.0 g (98.3%) of a white powder. 1H NMR: (200 MHz, CDCl3): δ 7.47 (d, 2H, J=8.2 Hz), 7.10 (d, 2H, J=8.2 Hz), 4.02 (q, J=7.0 Hz), 2.79-2.65 (m, 2H), 2.28 (s, 3H), 2.25 (t, 2H, J=7.0 Hz), 1.16 (t, 3H, J=7.0 Hz)...
example 3
Synthesis of a dT10 Oligonucleotide Sequence Conjugated to Bifunctional Linker (13)
[0215] A dT10 oligonucleotide sequence was assembled on an Applied Biosystems model 391 synthesizer on a 1 μmol scale on a CPG 500 dT-support. The synthesis protocol supplied by the instrument manufacturer was followed with the exception that a 0.25 M solution of 4,5-dicyanoimidazole (DCI) was used as the activator solution. The amidite (13) was used in an additional synthesis cycle on the support as a 0.1 M solution without further modifications of the cycle. The resulting oligonucleotide was obtained in the trityl-on mode and subjected to treatment with concentrated aqueous ammonia for 24 hours at room temperature, which cleaved the oligonucleotide from the support and removed the N-Fmoc protective group of the linker in one step. The crude product was 91.9% pure as analyzed by anion-exchange chromatography and found to contain 1.2% dT10 indicating a coupling efficiency greater than 98%. The produc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| emission wavelengths | aaaaa | aaaaa |
| emission wavelengths | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com