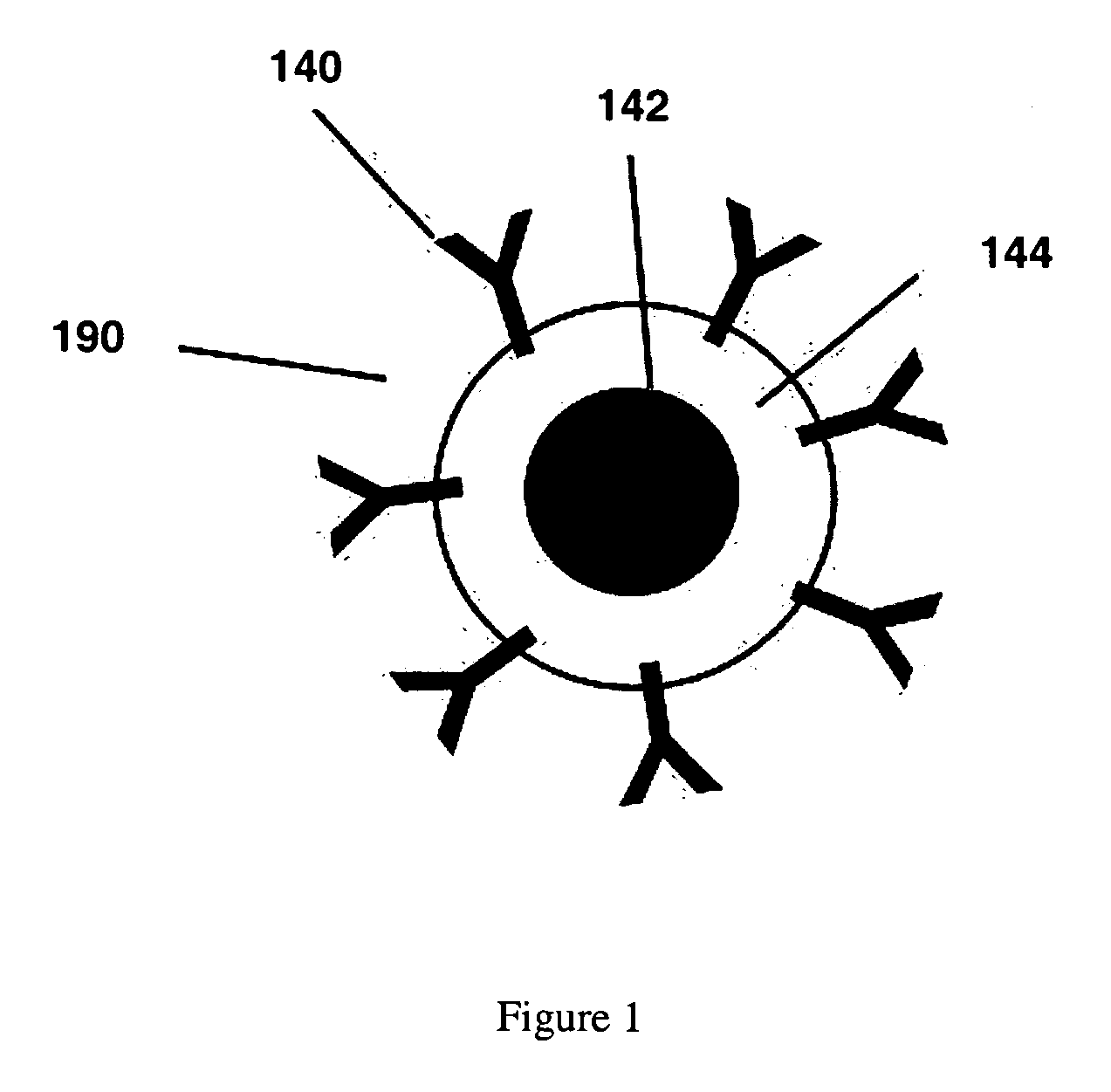
Magnetic nanoparticle compositions, and methods related thereto
a technology of magnetic nanoparticles and compositions, applied in the direction of magnetic bodies, drug compositions, metabolic disorders, etc., can solve the problem of inability to produce biocompatible magnetic nanoparticle compositions with enhanced homogeneity, and achieve the effect of enhancing homogeneity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Precipitation and Analysis of Iron Oxide (Lot 146-T)
[0093] 54.1 g (0.2 mol) of FeCl3×6H2O and 31.4 g (0.158 mol) of FeCl2×4H2O were dissolved in 300 milliliter (ml) of de-ionized water. The solution of the iron salts was stirred with a mechanical stirrer at 500 revolutions per minute (rpm) in a 1 L two neck flask at room temperature. 220 ml of 25% ammonium hydroxide was added to the solution with a peristaltic pump over a period of 30 minutes (min). After addition of the ammonium hydroxide, the stirring process was continued for 10 min. The iron oxide suspension was purified via centrifugation in two 500 ml centrifuge beakers at 1100 rpm for 10 min. After removal of the supernatant, the iron oxide pellet was re-suspended two times in 200 ml of de-ionized water. The re-suspension was centrifuged three more times for successive neutralization of the iron oxide suspension. The iron oxide suspension was centrifuged again in two 500 ml beakers at 1100 rpm for 10 min. After removal of th...
example 2
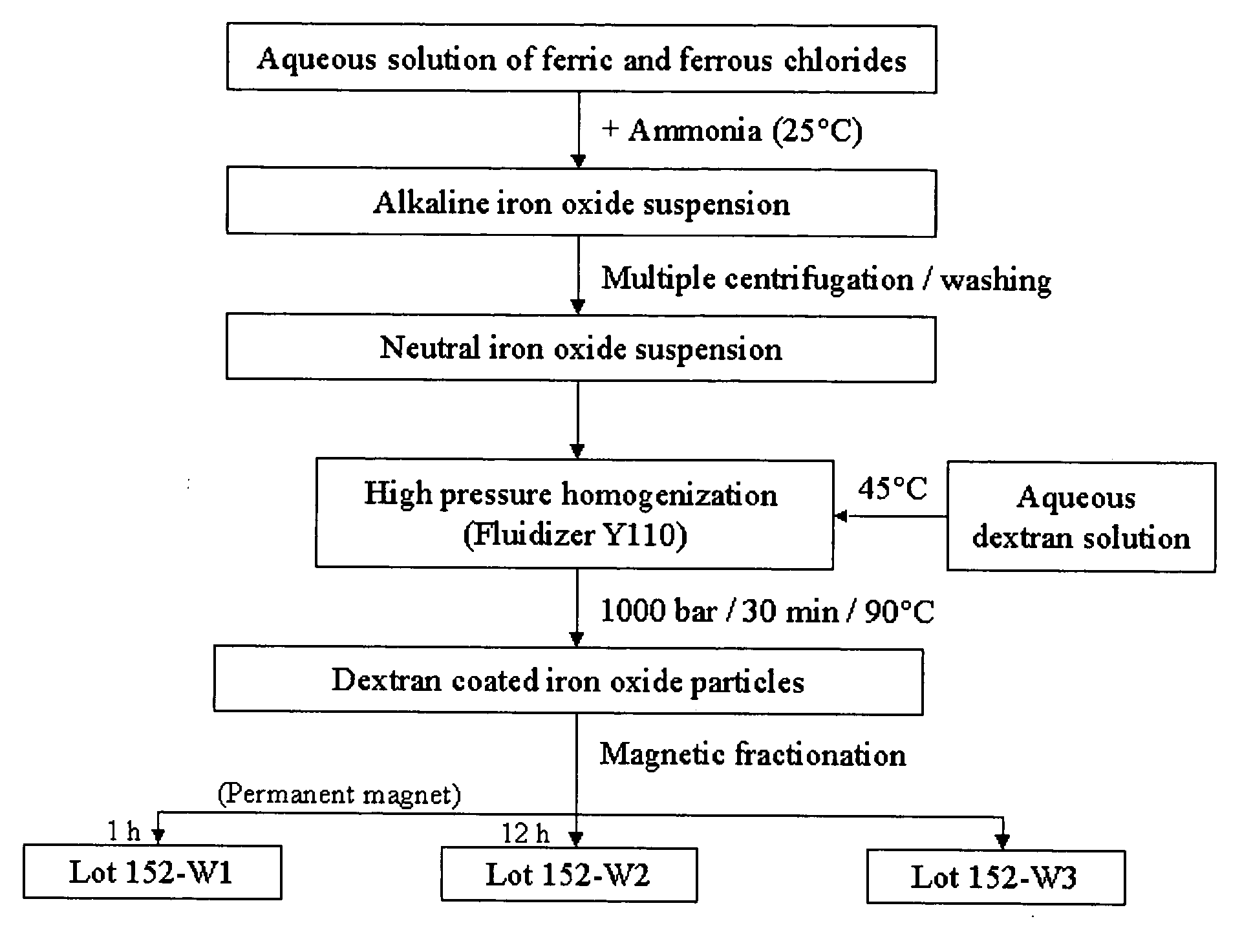
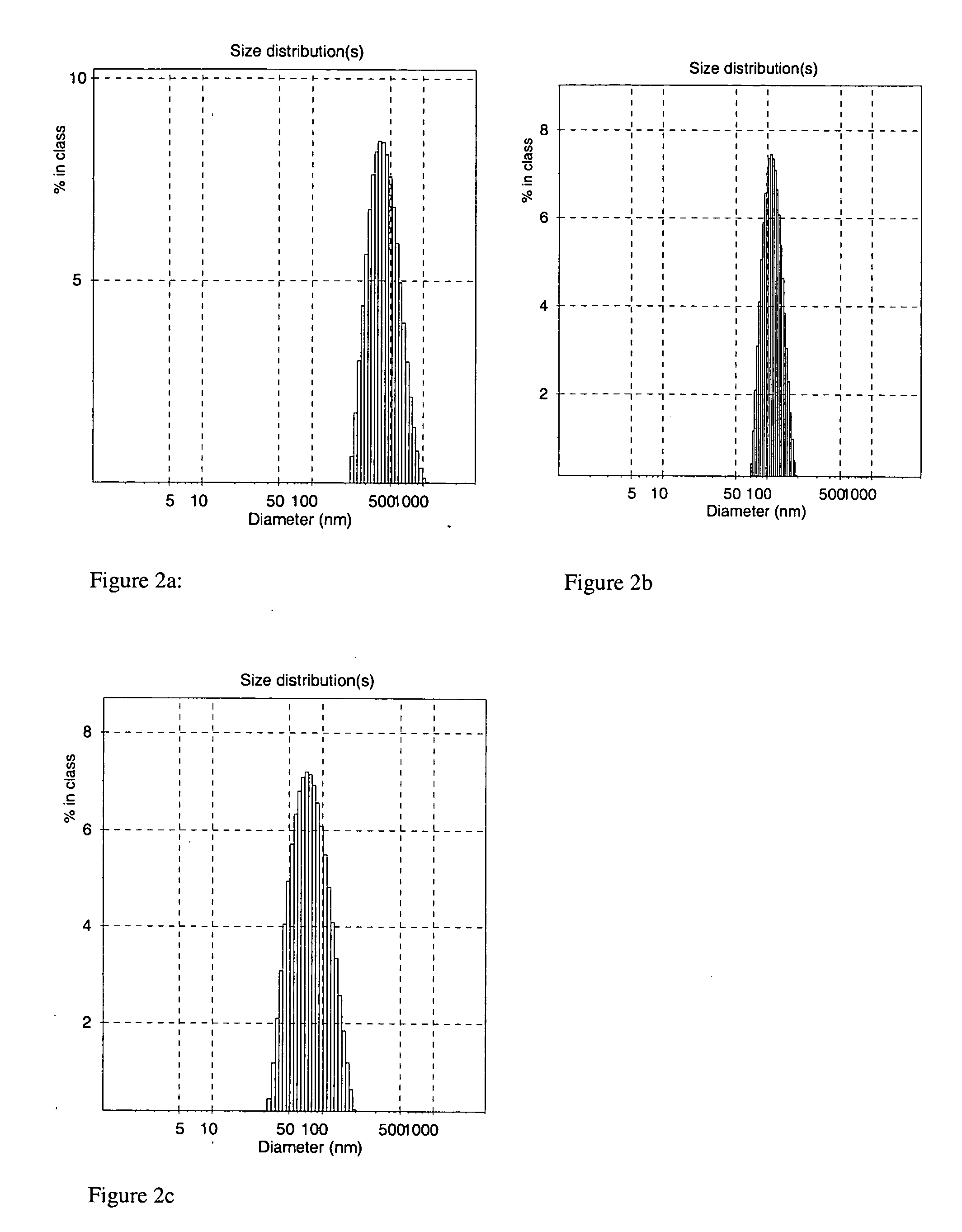
Synthesis and Characterization of Dextran-Coated Nanoparticles (Lot 152-W)
[0104] 21 g of dextran (molecular weight (MW)=40.000 Daltons, available from Carl Roth, GmbH, Karlsruhe, Germany) was dissolved in 100 ml of de-ionized water. 75 ml of iron oxide suspension from Lot 146-T (concentration=45 mg / ml) was homogenized in a high-pressure-homogenizer (Fluidizer Y110, Microfluidics, Inc., Newton, Mass.) at 500 bar for 7 min. This is a pressured, air driven plunger pump that drives the fluid through a 100 micrometer (μm) diamond gap in order to force a high shear stress. The pre-heated dextran solution (about 45° C.) was added to the suspension, warmed in the homogenizer to 90° C. at 1000 bar and processed at 1000 bar for 30 min at a temperature between 87° C.-92° C. After cooling to room temperature, the nanoparticle suspension was transferred into a crystallizing dish (diameter: 12 centimeter (cm)) and placed on a permanent magnet plate for 1 hour. Then, the supernatant was transferr...
example 3
PEG-Carboxylation of Dextran-Coated Nanoparticles (Lot 202-G)
[0108] 6 ml of a suspension of dextran-coated nanoparticles of 152-W-type (example 2) with a particle concentration of 25 mg / ml in 0.1 M β-morpholino ethanesulfonic acid hydrate buffer (pH=6.3) was treated with increasing amounts of carbodiimide activated 3,6-dioxaoctanedioic acid to yield the lots of nanoparticles 202-G1-202-G4 with various PEG-COOH densities, as presented in Table V.
TABLE VTHEORETICAL PEG-COOH DENSITY OF MAGNETICNANOPARTICLESLot ID. ofAmounts of EDC, and 3,6-PEG-COOHdioxaoctanedioic acid [mgTheoretical PEG-COOHparticlesper 150 mg particle]density [nmol / mg]202-G118625202-G23.6125202-G30.7225202-G40.1445
[0109] Equal amounts (as shown in Table V) of 1-(3-dimethyl-aminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and 3,6-dioxaoctanedioic acid were dissolved in 1.5 ml of 0.5 M β-morpholino ethanesulfonic acid hydrate buffer (pH=6.3), incubated at 50° C. for 10 min, and added to the particle suspension. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com