Gel composition and methods
a technology of gel and composition, applied in the field of gel composition, can solve the problems of not always satisfying the demand for biodegradable implants, reluctance of patients to accept such implants or drug delivery systems, and important limitations of their use in the body of various animals, so as to reduce the loading rate of beneficial agents, and improve the effect of biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0141] Lysozyme particles were made by spray drying 50% sucrose and 50% chicken lysozyme (on a dry weight basis).
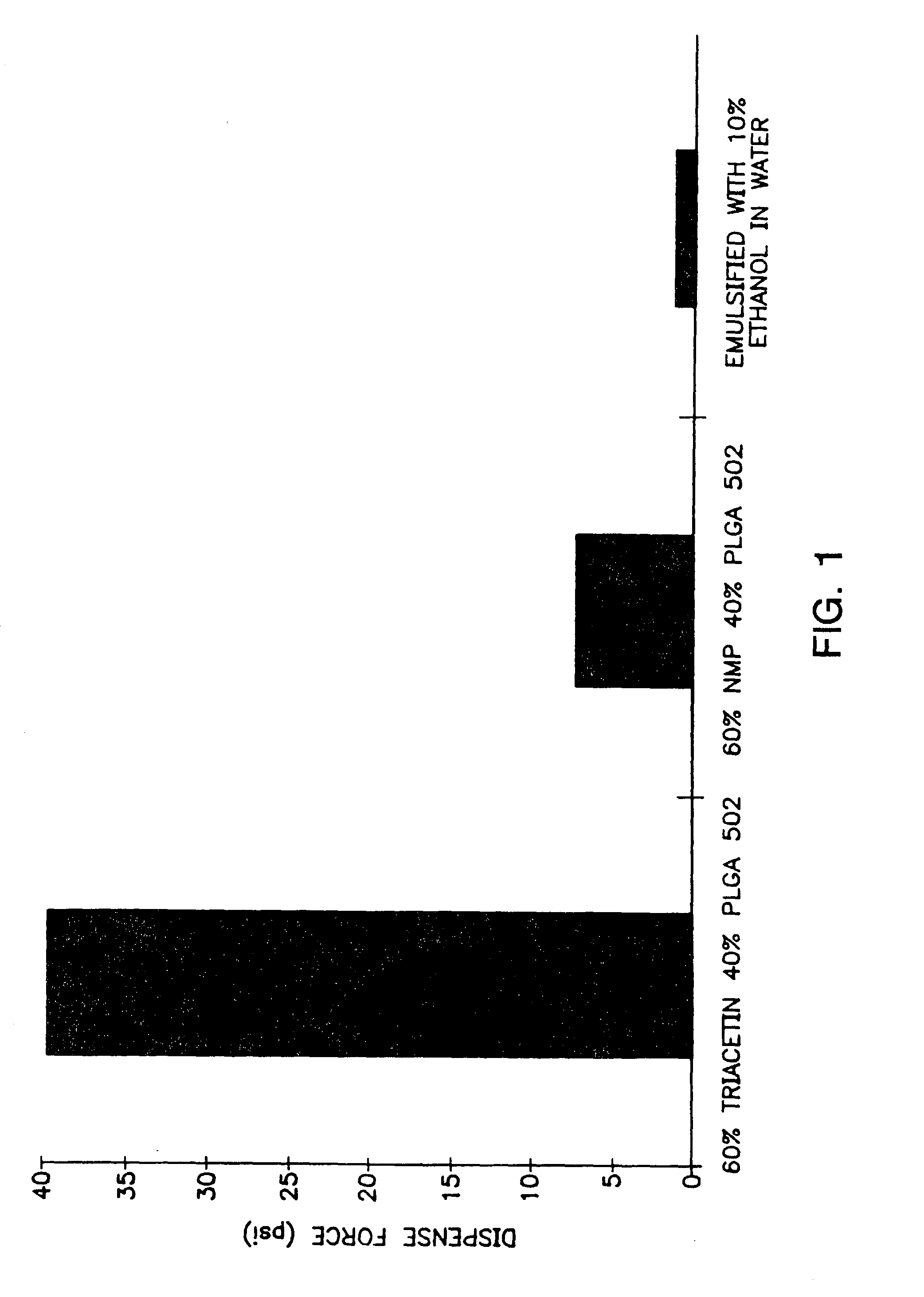
[0142] A viscous gel material was prepared by heating 60% by weight of triacetin with 40% by weight of a 50:50 lactic acid:glycolic acid copolymer to 37° C. overnight. The viscous gel was allowed to cool to room temperature. The lysozyme particles were added to the viscous gel in a ratio of 20:80 lysozyme particles:gel (by weight). The combination was mixed for 5 minutes. Immediately prior to use, a 10% ethanol, 90% isotonic saline solution was added as the emulsifying agent. The emulsifying agent comprised ⅓ of the total injectable depot gel composition. The prepared compositions were suitable for injection.
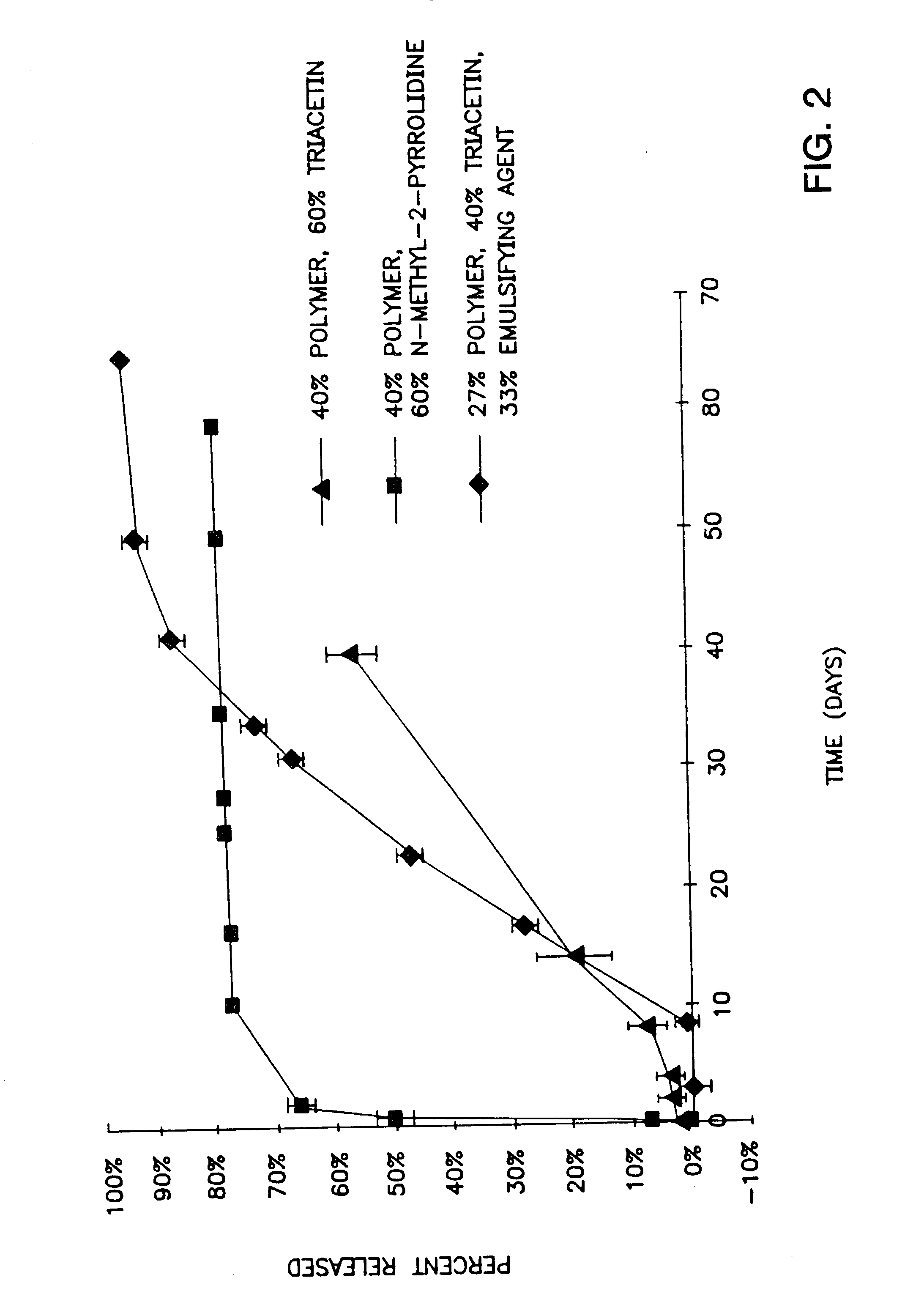
[0143]FIG. 2 shows the in vitro release rates obtained from the compositions described with regard to FIG. 1. The gel prepared from 40% by weight of a 50:50 lactic acid:glycolic polymer and 60% by weight triacetin is thick and thus difficult to inject but shows litt...
example 2
hGH Particle Preparation
[0144] Human growth hormone (hGH) particles (optionally containing zinc acetate) were prepared as follows:
[0145] hGH solution (5 mg / ml) solution in water (BresaGen Corporation, Adelaide, Australia) was concentrated to 10 mg / mL using a Concentration / Dialysis Selector diafiltering apparatus. The diafiltered hGH solution was then washed with 5 times volume of tris or phosphate buffer solution (pH 7.6). Particles of hGH were then formed by spray drying or lyophilization using conventional techniques. Phosphate buffer solutions (5 or 50 mM) containing hGH (5 mg / mL) and various levels of zinc acetate (0 to 30 mM) were spray-dried using a Yamato Mini Spraydryer set at the following parameters:
Spray Dryer ParameterSettingAtomizing Air2psiInlet Temperature120° C.Aspirator Dial7.5Solution Pump2-4Main Air Valve40-45psi
[0146] hGH particles having a size range between 2-100 microns were obtained. Lyophilized particles were prepared from tris buffer solutions (5 or 50 ...
example 3
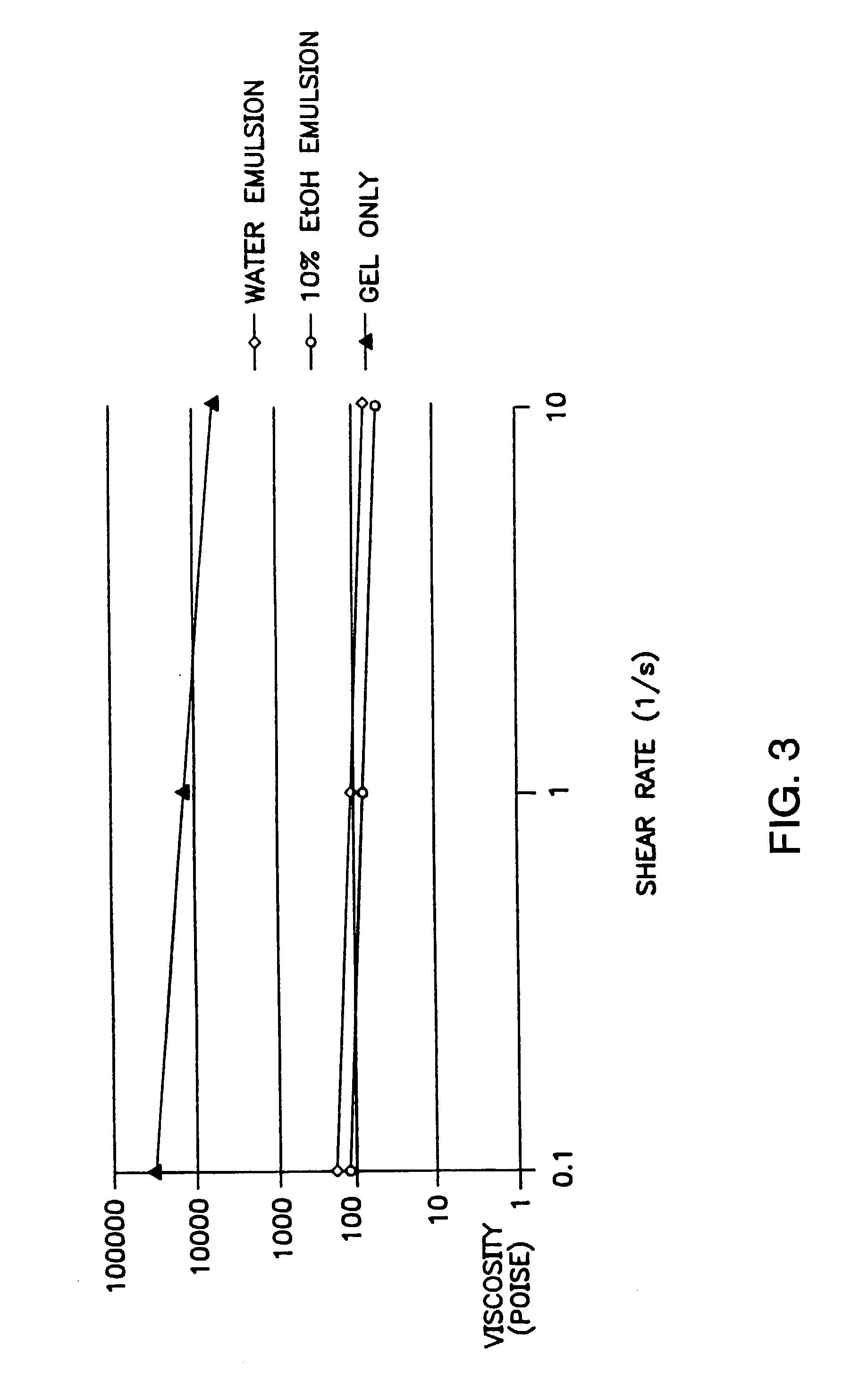
[0151] Lysozyme from chicken egg white (Sigma Chemical Co., St Louis, Mo.) in vitro release studies were used to test different vehicle formulations with the highly water soluble solvent NMP and the less soluble solvents triacetin and benzyl benzoate useful in the present invention. A depot gel formulation was dispensed from a 3 cc disposable syringe and weighed onto a Delrin™ cup platform or a 250 μ mesh 1 square inch polypropylene screen. Then, the cup or screen containing a depot gel formulation was immersed into a plastic vial containing 10 mL of receptor buffer. A snap-on lid was placed onto the plastic vial to prevent evaporation. The vial containing the depot gel formulation was immersed into a Haake shaking water bath equilibrated to 37° C. At each time point, forceps were used to transfer Delrin™ cup platforms or polypropylene screen platforms containing depot gel formulations to new plastic vials containing 10 mL of receptor buffer. Disposable tra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| miscibility | aaaaa | aaaaa |
| miscibility | aaaaa | aaaaa |
| miscibility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com