Plant bioreactors
a bioreactor and plant technology, applied in the field of plants as bioreactors, can solve the problems of unfavorable immune response, unfavorable steps involving purification or processing, and low protein production cost, and achieve the effect of enhancing the effect of an immune respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
AFP Gene Construction and Expression
[0162] To maximize potential AFP production at the translational level, the native 5′-untranslated region of the fish cDNA was replaced with the 5′-untranslated leader region of the tobacco mosaic virus (TMV) (Richards et al., 1977 & 1978; Sleat et al., 1988). The fish signal peptide sequence was also replaced with that of the tobacco pathogenesis-related protein 1b (PR-1b) (Cornelissen et al., 1986; Sijmons et al., 1990; Denecke et al., 1990). Oligonucleotide cassettes were designed: three oligonucleotides, #1091 (SEQ ID NO:1), #1092 (SEQ ID NO:4), and #1309 (SEQ ID NO:2), which would hybridize to generate the complete TMV leader sequence (TMV Cassette: FIG. 10A); and five oligonucleotides #4361-#4365 (SEQ ID NOS: 3, 5, 6, 8 and 9, respectively) which in concert with oligonucleotides #1091 and #1092 (SEQ ID NOS:1 and 4) would hybridize to generate a linked TMV leader and PR-1b signal peptide DNA sequence (TMV-PR Cassette: FIG. 10B). The 5′ end o...
example 2
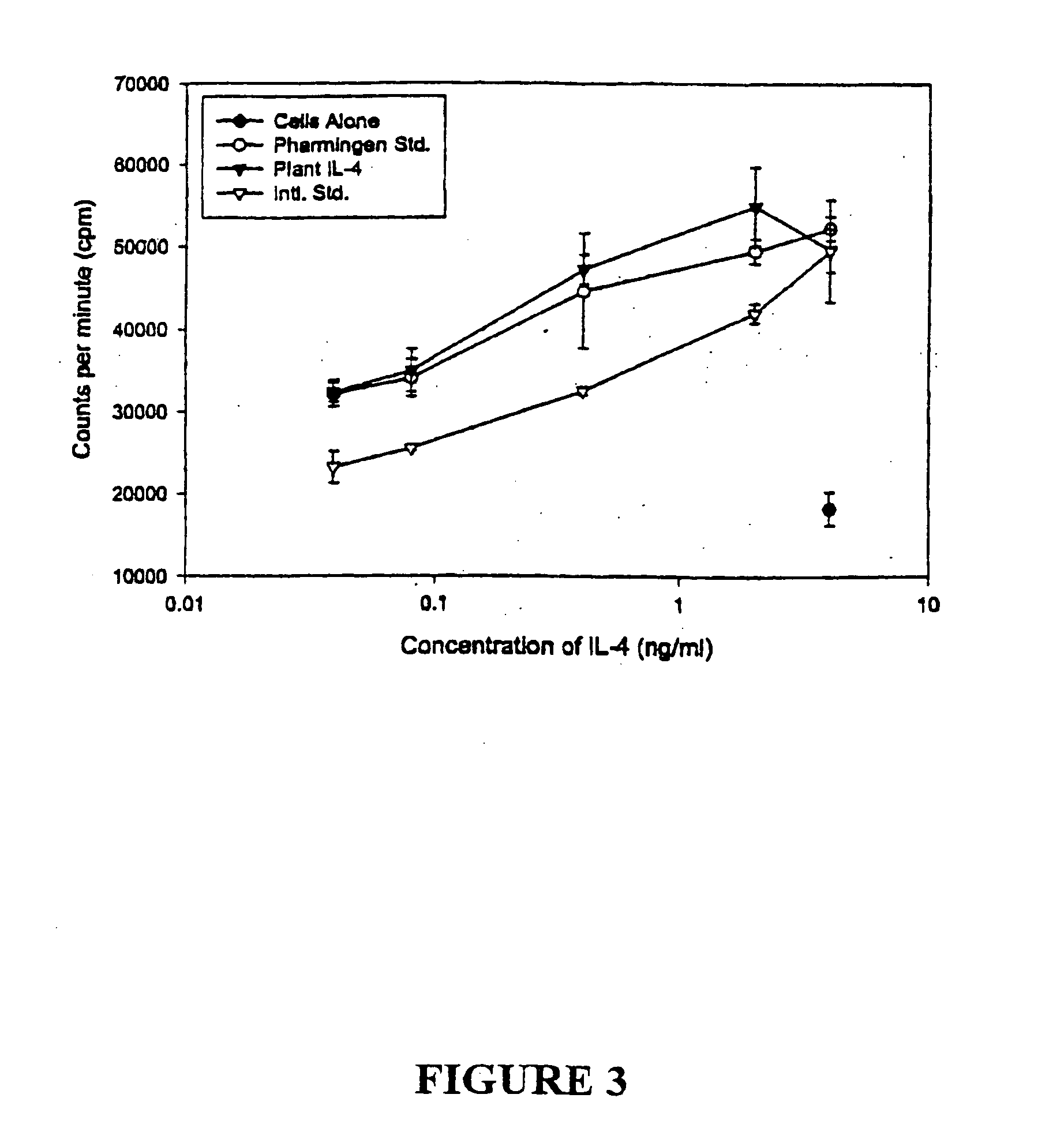
IL-4 Expression and Determination of Biological Activity
[0177] Mouse IL-4 is a glycoprotein of 140 amino acid residues including a putative 20-amino acid signal peptide, which has a molecular weight of 19 to 21 kDa, depending on the degree of glycosylation (Proc. Natl. Acad. Sci. USA 83: 2061-2065, 1986). Mouse IL-4 cDNA was first amplified with PCR using the primers:
(SEQ ID NO:13)5′-AAT CTCGAGCATATC CAC GGATGCGAC-3′and(SEQ ID NO:14)5′-ATAGGTACCGTAATCCATTTGC ATGATGC-3′,
and the PCR product encoding the full protein was isolated and ligated to a signal peptide encoding sequence from peanut peroxidase and the KDEL sequence (SEQ ID NO:22). To facilitate subsequent purification of the recombinant product from plant cells, a DNA sequence encoding 6 consecutive histidines was also included in the reconstituted IL-4 gene (SEQ ID NO:21). The reconstituted IL-4 cDNA was then used to replace the β-glucuronidase (GUS) gene in plasmid pTRL2-GUS composed of a CaMV35S promoter with a double en...
example 3
Interleukin-10 (IL-10) Expression and Determination of Biological Activity
[0185] Full length mRNA encoding human IL-10 (hIL-10) is 1601 nucleotides long, with a short 5′UTR of 30 nucleotides (nt) and a long 3′UTR of 1037 nt. The hIL-10 coding sequence is 534 base pairs long and codes for a 178 amino acid preprotein with a 18 amino acid secretory signal (Vieira et al., 1991). The full length human IL-10 cDNA is isolated from polyA RNA by RT-PCR and fully sequenced to ensure fidelity. In order to maximize expression levels and transgene protein production, the IL-10 cDNA is examined for codon usage patterns and optimized as necessary (Perlak et al., 1991).
[0186] Since ER retention signals can increase the concentration of disulfide bonded transgene proteins, therefore the KDEL ER retention motif may be added to the IL-10 cDNA using site directed mutagenesis (see results in FIG. 5C). For comparative purposes an identical synthetic gene, without the KDEL signal, is prepared and used i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com