Water soluble paclitaxel derivatives
a technology of paclitaxel and derivatives, applied in the field of pharmaceutical compositions, can solve the problems of prolonged drug exposure to tumor cells and tumor accumulation, and achieve the effects of novel drug activity, improved efficacy and controlled release, and surprising anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Poly-glutamic Acid-Paclitaxel (PG-TXL)
[0092] The present example concerns a first study involving the conjugation of paclitaxel to a water-soluble polymer, poly (1-glutamic acid) (PG) and the efficacy of the preparation against a variety of tumors in mice and rats. The potential of water-soluble polymers used as drug carriers is well established (Kopecek, 1990; Maeda and Matsumura, 1989).
Synthesis of Poly-Glutamic Acid-Paclitaxel (PG-TXL)
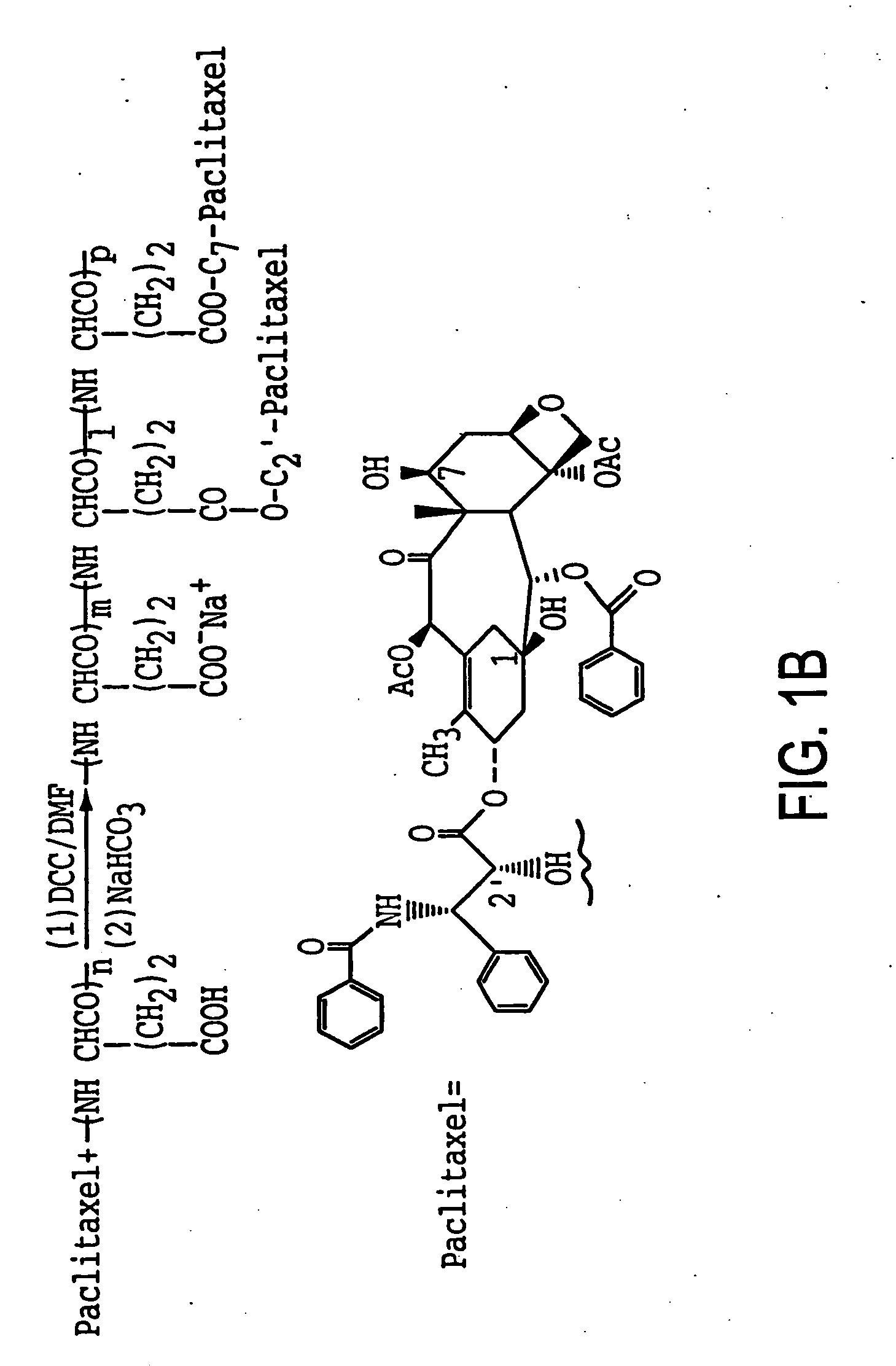
[0093] PG was selected as a carrier for paclitaxel because it can be readily degraded by lysosomal enzymes, is stable in plasma and contains sufficient functional groups for drug attachment. Several antitumor drugs, including Adriamycin (Van Heeswijk et al., 1985; Hoes et al., 1985), cyclophosphamide (Hirano et al., 1979), Ara-C (Kato et al., 1984) and melphalan (Morimoto et al., 1984) have been conjugated to PG. However, poly-aspartic acid may be conjugated to anti-tumor drugs using the reaction scheme described herein for PG-TXL.
[0094] The re...
example 2
DTPA-Paclitaxel
Synthesis of DTPA-Paclitaxel:
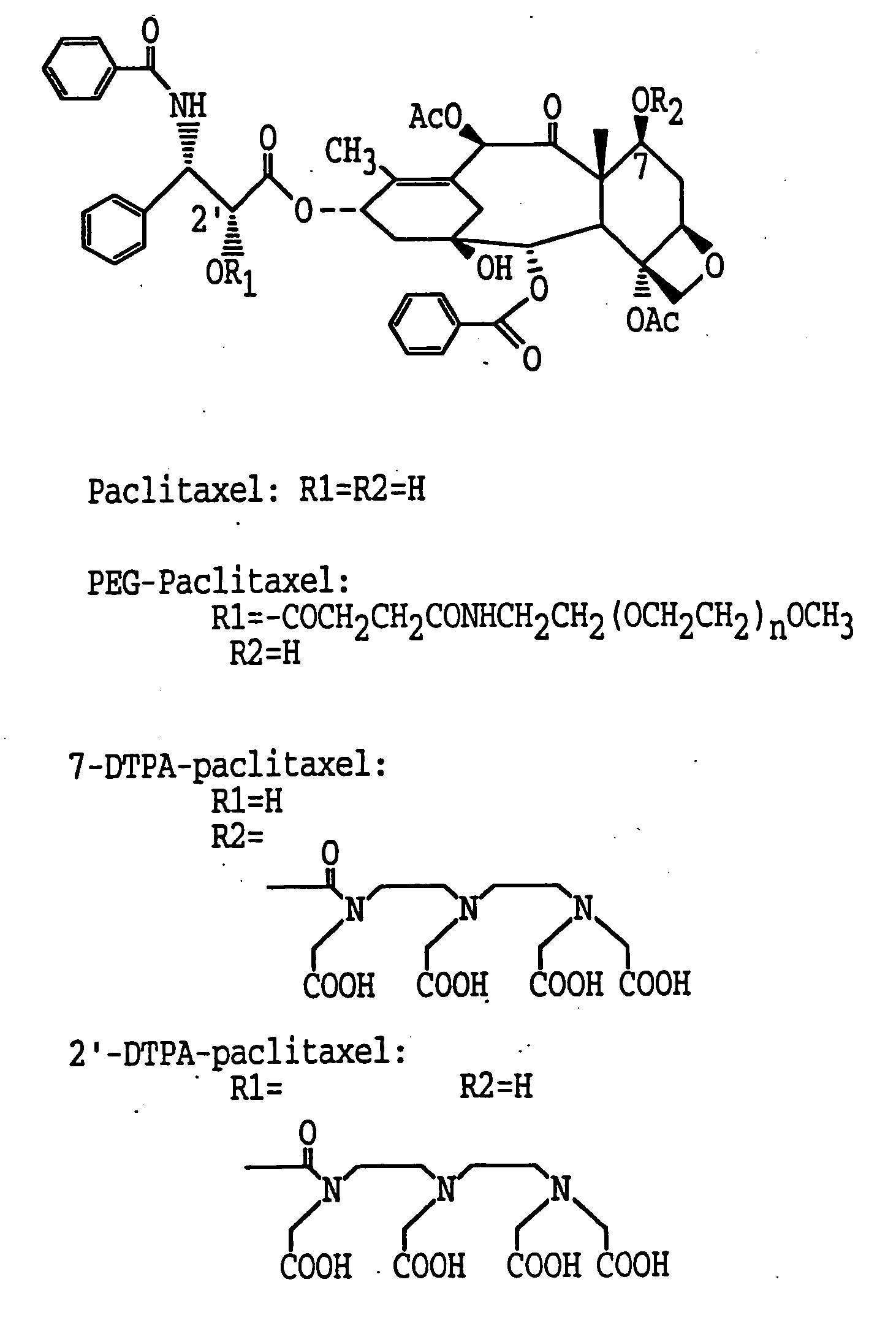
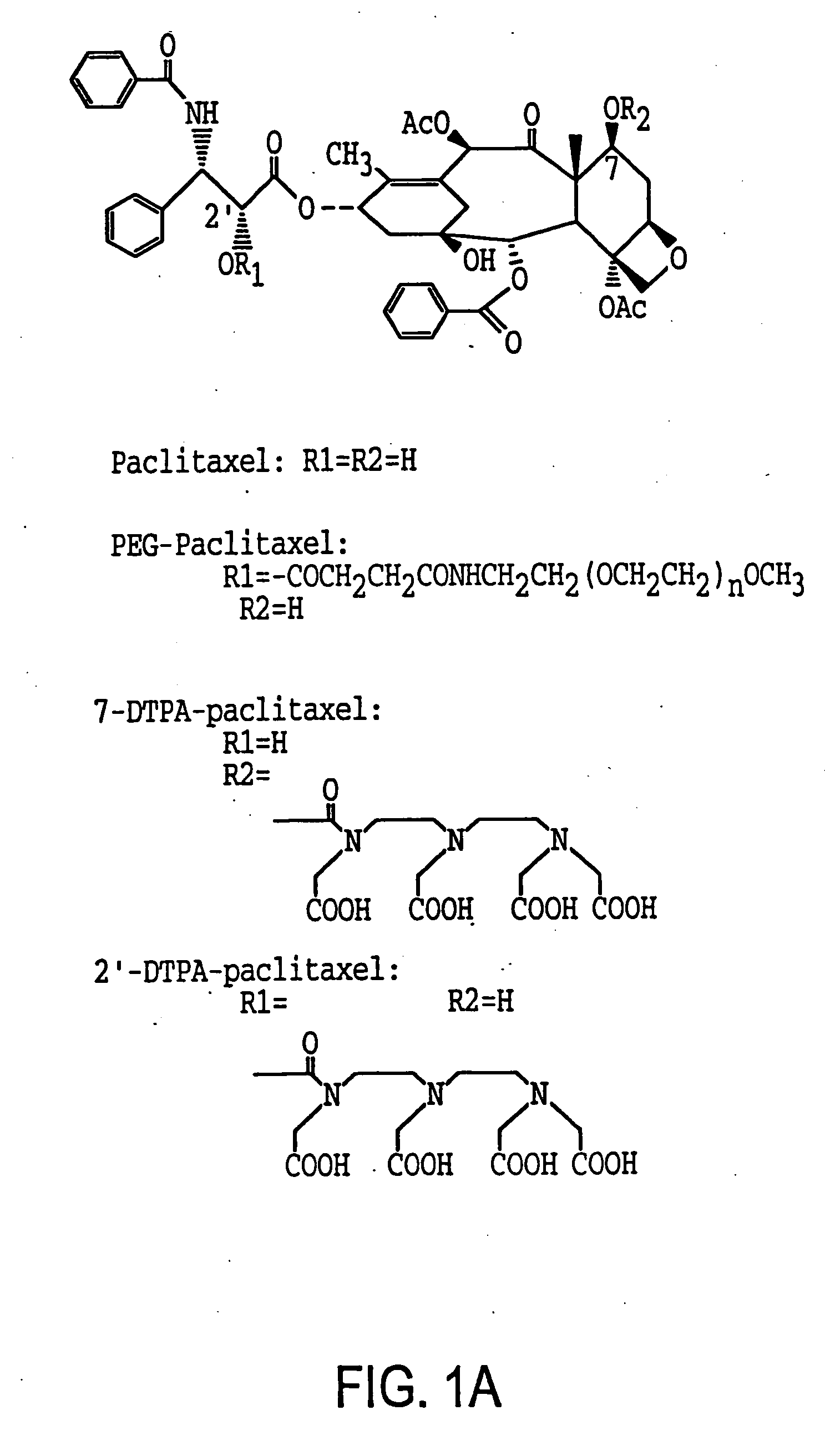
[0117] To a solution of pacitaxel (100 mg, 0.117 mmol) in dry DMF (2.2 ml) was added diethylenetriaminepentaacetic acid anhydride (DTPA A) (210 mg, 0.585 mmol) at 0° C. The reaction mixture was stirred at 4° C. overnight. The suspension was filtered (0.2 μm Millipore filter) to remove unreacted DTPA anhydride. The filtrate was poured into distilled water, stirred at 4° C. for 20 min, and the precipitate collected. The crude product was purified by preparative TLC over C18 silica gel plates and developed in acetonitrile / water (1:1). Paclitaxel had an Rf value of 0.34. The band above the paclitaxel with an Rf. value of 0.65 to 0.75 was removed by scraping and eluted with an acetonitrile / water (1:1) mixture, and the solvent was removed to give 15 mg of DTPA-paclitaxel as product (yield 10.4%): mp: >226° C. dec. The UV spectrum (sodium salt in water) showed maximal absorption at 228 nm which is also characteristic for paclitaxel. Mass spect...
example 3
Polyethylene glycol-Paclitaxel
Synthesis of Polyethylene Glycol-Paclitaxel (PEG-Paclitaxel)
[0128] The synthesis was accomplished in two steps. First 2′-succinyl-paclitaxel was prepared according to a reported procedure (Deutsch et al., 1989). Paclitaxel (200 mg, 0.23 mmol) and succinic anhydride (288 mg, 2.22 mmol) were allowed to react in anhydrous pyridine (6 ml) at room temperature for 3 h. The pyridine was then evaporated, and the residue was treated with water, stirred for 20 mm, and filtered. The precipitate was dissolved in acetone, water was slowly added, and the fine crystals were collected to yield 1 80 mg 2′-succinyl-paclitaxel. PEG-paciitaxel was synthesized by an N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) mediated coupling reaction. To a solution of 2′-succinyl-paclitaxel (160 mg, 0.18 mmol) and methoxypolyoxyethylene amine (PEG-NH2, MW 5000, 900 mg, 0.18 mmol) in methylene chloride was added EEDQ (180 mg, 0.72 mmol). The reaction mixture was stirred at roo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular mass | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com