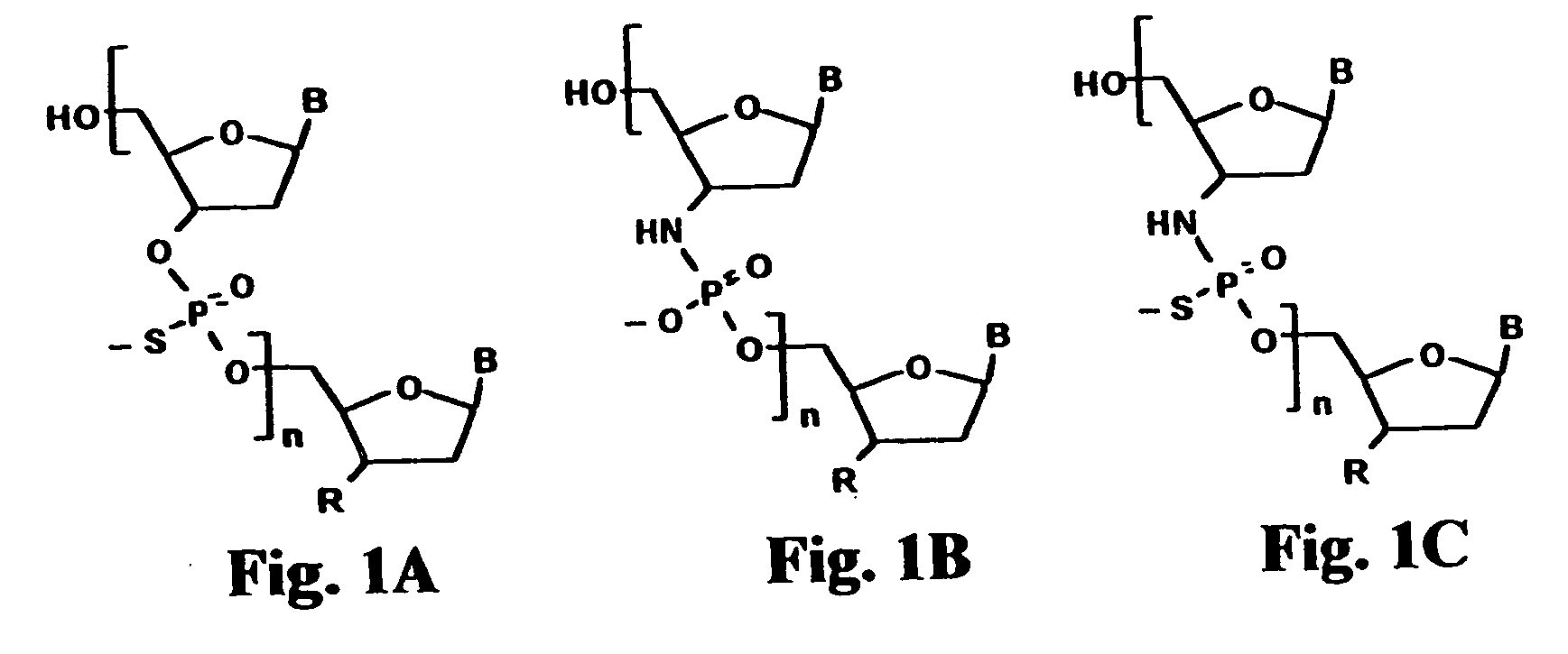
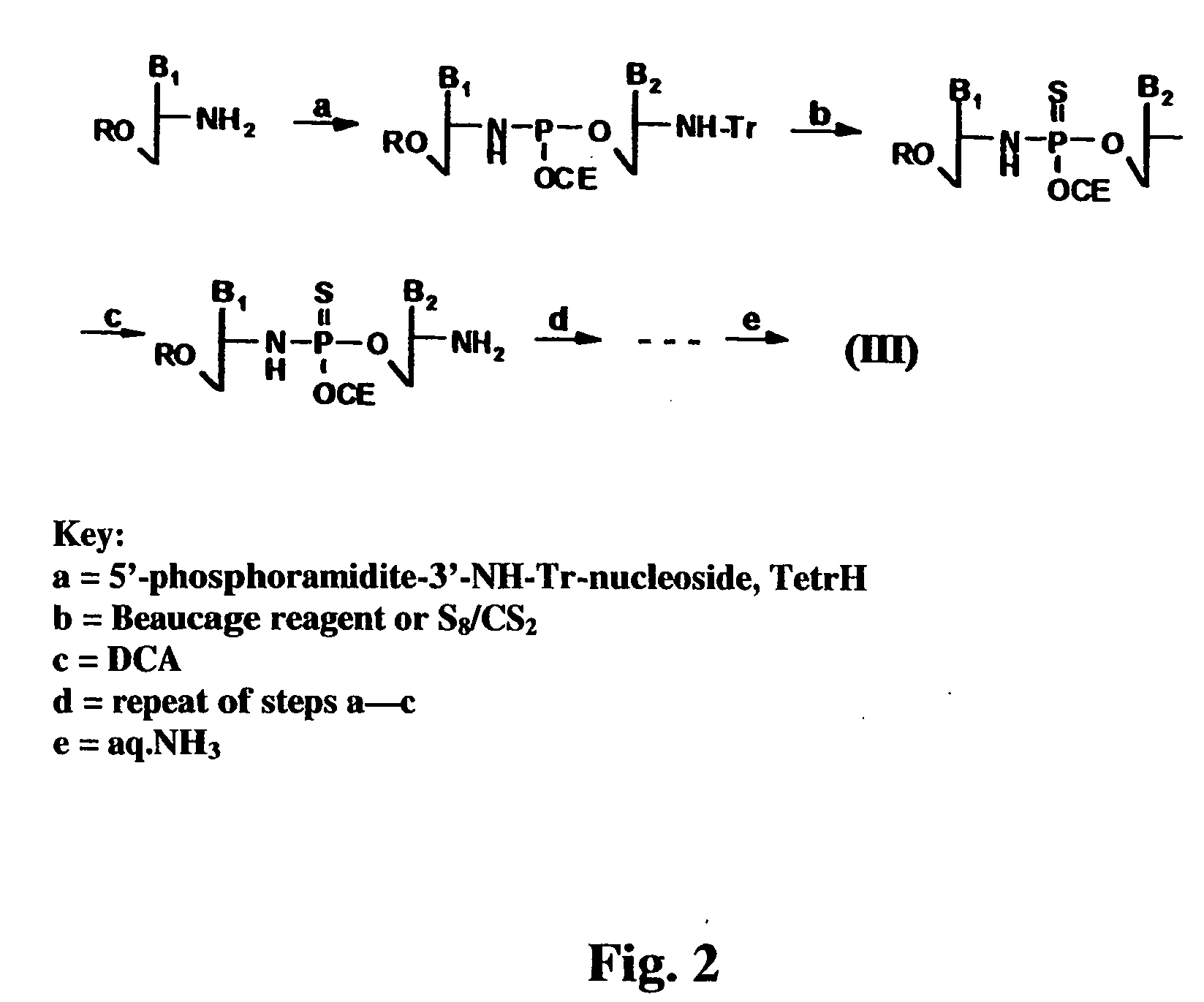
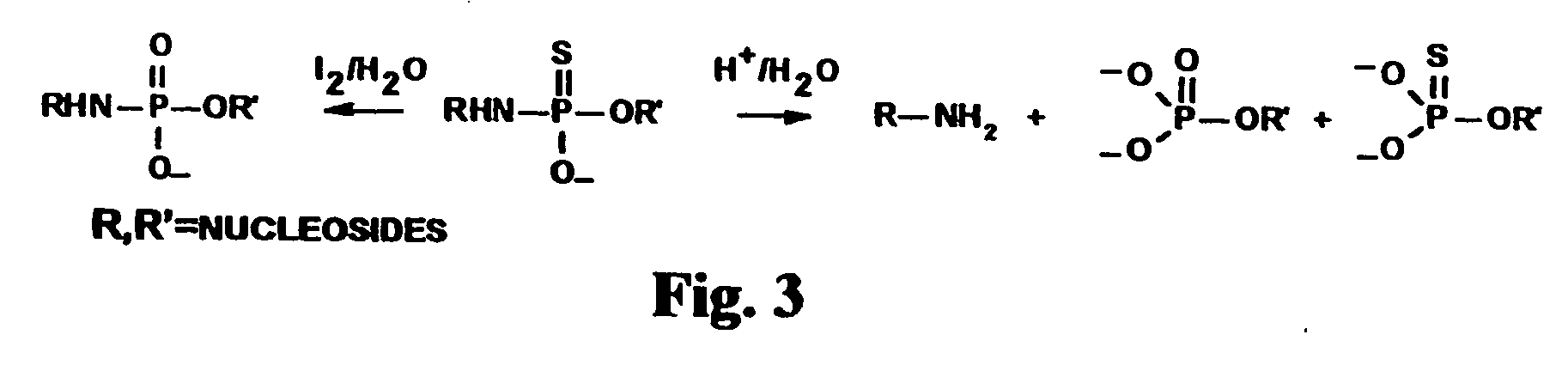
Oligonucleotide N3'-P5' thiophosphoramidates: their synthesis and use
a technology of thiophosphoramidates and oligonucleotides, applied in the field of oligonucleotides, can solve the problems of adversely affecting the pharmacological properties of some applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Methods
[0143]31P NMR spectra were obtained on a Varian 400 Mhz spectrometer. 31P NMR spectra were referenced against 85% aqueous phosphoric acid. Anion exchange HPLC was performed using a Dionex DX 500 Chromatography System, with a Pharmacia Bitotech Mono Q HR 5 / 5 or 10 / 16 ion exchange columns. Mass spectral analysis was performed by Mass Consortium, San Diego, Calif. MALDI-TOF analysis of oligonucleotides was obtained using a PerSpective Biosystems Voyager Elite mass spectrometer with delayed extraction. Thermal dissociation experiments were conducted on a Cary Bio 100 UV-Vis spectrometer.
[0144] All reactions were carried out in oven dried glassware under a nitrogen atmosphere unless otherwise stated. Commercially available DNA synthesis reagents were purchased from Glen Research (Sterling, Va.). Anhydrous pyridine, toluene, dichloromethane, diisopropylethyl amine, triethylamine, acetic anhydride, 1,2-dichloroethane, and dioxane were purchased from Aldrich (Milwaukee, Wis...
example 2
Synthesis of Arabino-fluorooligonucleotide N3′→P5′ Phosphoramidates
[0147] The solid phase synthesis of oligo-2′-arabino-fluoronucleotide N3′→P5′ phosphoramidates was based on the phosphoramidite transfer reaction employing the monomer building blocks—5′-(O-cyanoethyl-N,N′-diisopropylamino)-phosphoramidites of 3′-MMTr-protected-3′-amino-2′-ara-fluoro nucleosides. Preparation of the nucleoside monomers is depicted in Scheme 4.
[0148] Sugar precursor 1 (from Pfanstiehl) was converted into the α-1-bromo intermediate 2 with retention of sugar C-1 configuration. Compound 2 was then used, without isolation, for a SN2-type glycosylation reaction with silylated uracil and thymine bases, which resulted in formation of nucleosides 3. Stereo selectivity of the glycosylation reaction was quite high—more than 90% of the formed nucleoside 3 had the desired β-anomeric configuration, as was judged by 1H NMR analysis of the reaction mixture. The pure β-isomer of nucleoside 3 was isolated by crystal...
example 3
Synthesis of Oligonucleotide N3′→P5′ Thiophosphoramidates
[0149] Oligonucleotide N3′→P5′ thiophosphoramidates were prepared by the amidite transfer reaction on an ABI 394 synthesizer. The fully protected monomer building blocks were 3′-aminotrityl nucleoside-5′-(2-cyanoethyl-N,N-diisopropyl) phosphoramidite where nucleoside is 3′-deoxy-thymidine, 2′,3′-dideoxy-N2-isobutyryl-guanosine, 2′,3′-dideoxy-N6-benzoyl-adenosine or 2′,3′-dideoxy-N-benzoyl-cytidine. 5′-Succinyl-3′-aminotrityl-2′,3′-dideoxy nucleosides were coupled with an amino group containing long chain controlled pore glass (LCAA-CPG) and used as the solid support. The synthesis was performed in the direction of 5′ to 3′. The following protocol was used for the assembly of oligonucleotide N3′→P5′ thiophosphoramidates: (i) detritylation, 3% dichloroacetic acid in dichloromethane; (ii) coupling, 0.1 M phosphoramidite and 0.45 M tetrazole in acetonitrile, 25 sec; (iii) capping, isobutyric anhydride / 2,6-lutidine / THF 1 / 1 / 8 v / v / v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com