[0019]
Umbilical cord blood, the origin of therapeutic cells of the invention, is defined as blood taken from
umbilical vein connecting
placenta and a
fetus.
Umbilical cord blood is a natural by-product of
childbirth. The umbilical cord blood is much easier to obtain than general mesenchymal tissue like
bone marrow requiring several steps of operation and it is also very easy to find a donor because umbilical cord blood deposit industry is developing steadily and infra has already been established. In addition, umbilical cord blood-originated cells are the one that does not express
histocompatibility antigen HLA-DR (class II) which is the major cause of rejection after tissue- or
organ transplantation. Thus, these cells can minimize the immune response according to transplantation, for example rejection against
transplanted tissue or organ, suggesting that autologous or homologous umbilical cord blood transplantation is very useful and effective.
[0022]A composition of the present invention contains one or more cells selected from a group consisting of monocytes such as autologous hematopoietic stem cells and mesenchymal stem cells derived from the umbilical cord blood, mesenchymal stem cells derived from the umbilical cord blood, and mesenchymal stem cells sub-cultured and amplified from the mesenchymal stem cells. The mesenchymal stem cells derived from the umbilical cord blood are multipotent, unlike the typical stromal cells of
bone marrow, suggesting that they are able to be differentiated into mesenchymal tissues such as bone,
cartilage,
adipose tissue,
muscle,
tendon, etc., under a proper condition.
Umbilical cord blood derived mesenchymal stem cells have ability of self-renewal, suggesting that they are capable of proliferating under a proper condition, and might exhibit anti-
inflammation activity when transplanted. Compared with those cells originated from mesenchymal stem cells derived from general mesenchymal tissues such as bone marrow,
muscle and
skin, these more primitive cells have far much excellent
cell proliferation, and differentiation, and regulation substance secreting capacity.
[0025]The cell suspension is distributed into glass- or plastic ampoules for
deep freezing, and then the ampoules are sealed and put in a deep freezer programmed to proper temperature. At this time, it is preferred to use a freeze-program allowing −1° C. / min of temperature change in order to reduce
cell damage during thawing.
[0028]According to the present invention,
cell concentration is 1.0×105-1.0×109 cells / Ml, and 1.0×106-1.0×108 cells / Ml is more preferred and 1.0×107 cells / Ml is most preferred. A composition of the present invention is preferably intratracheally-administered as close to lung tissues as possible, to increase
therapeutic effect by improved
accessibility, compared with the conventional
cell transplantation using intravenous injection.
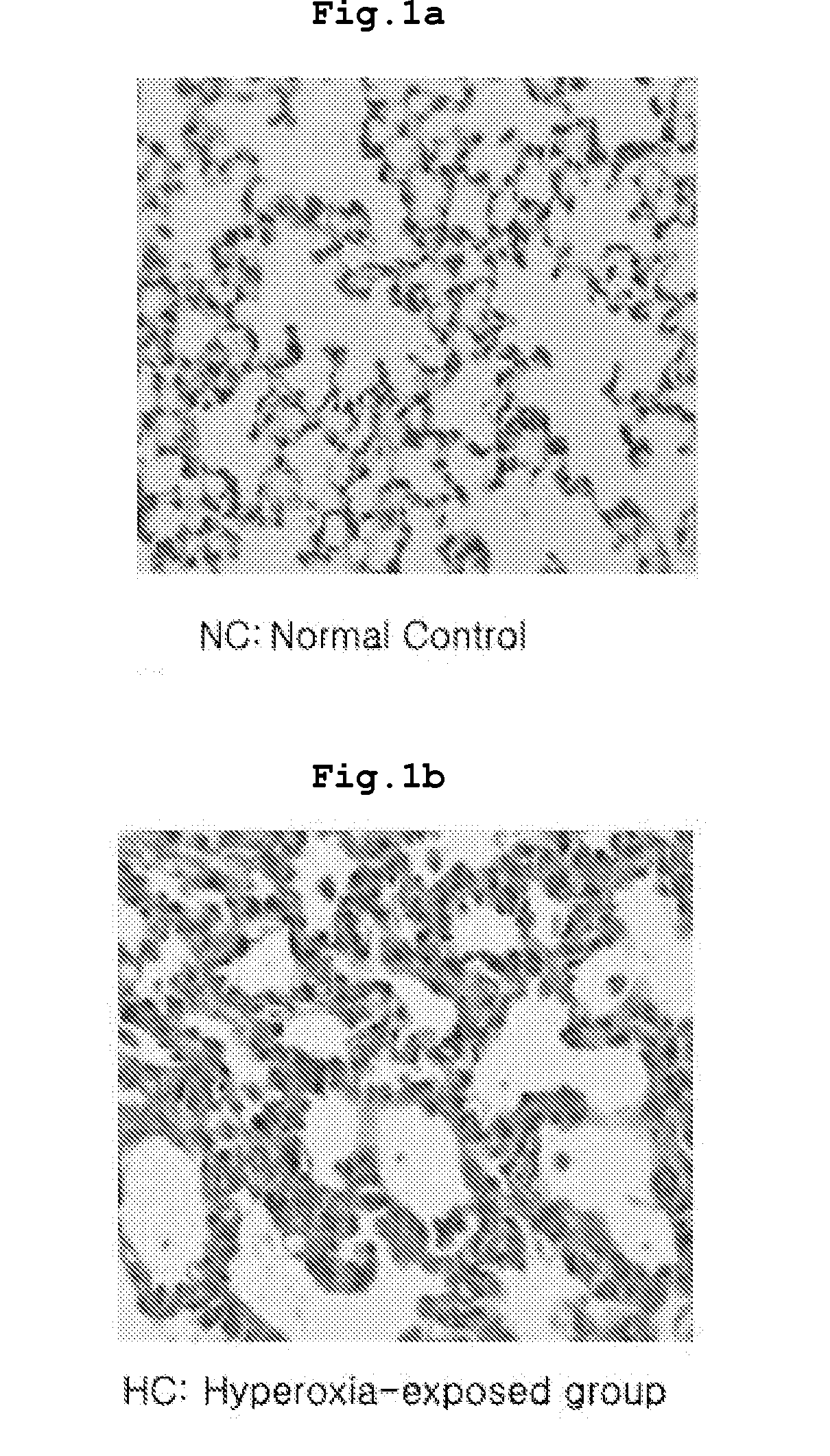
[0029]The present inventors centrifuged the extracted umbilical cord blood to separate monocytes, then the separated cells were cultured with a proper density in a
culture vessel. When the cells were grown to proper density, sub-cultures were performed. The present inventors established a
bronchopulmonary dysplasia model in neonatal rats by administering high-concentrated
oxygen continuously from the birth.
Bronchoalveolar lavage were obtained and the lungs were extracted from the
animal model and stained. As a result, rats with bronchopulmonary
dysplasia exhibited increased
respiratory rate, poor
weight gain, and chronic inflammatory reactions with monocytic infiltration and
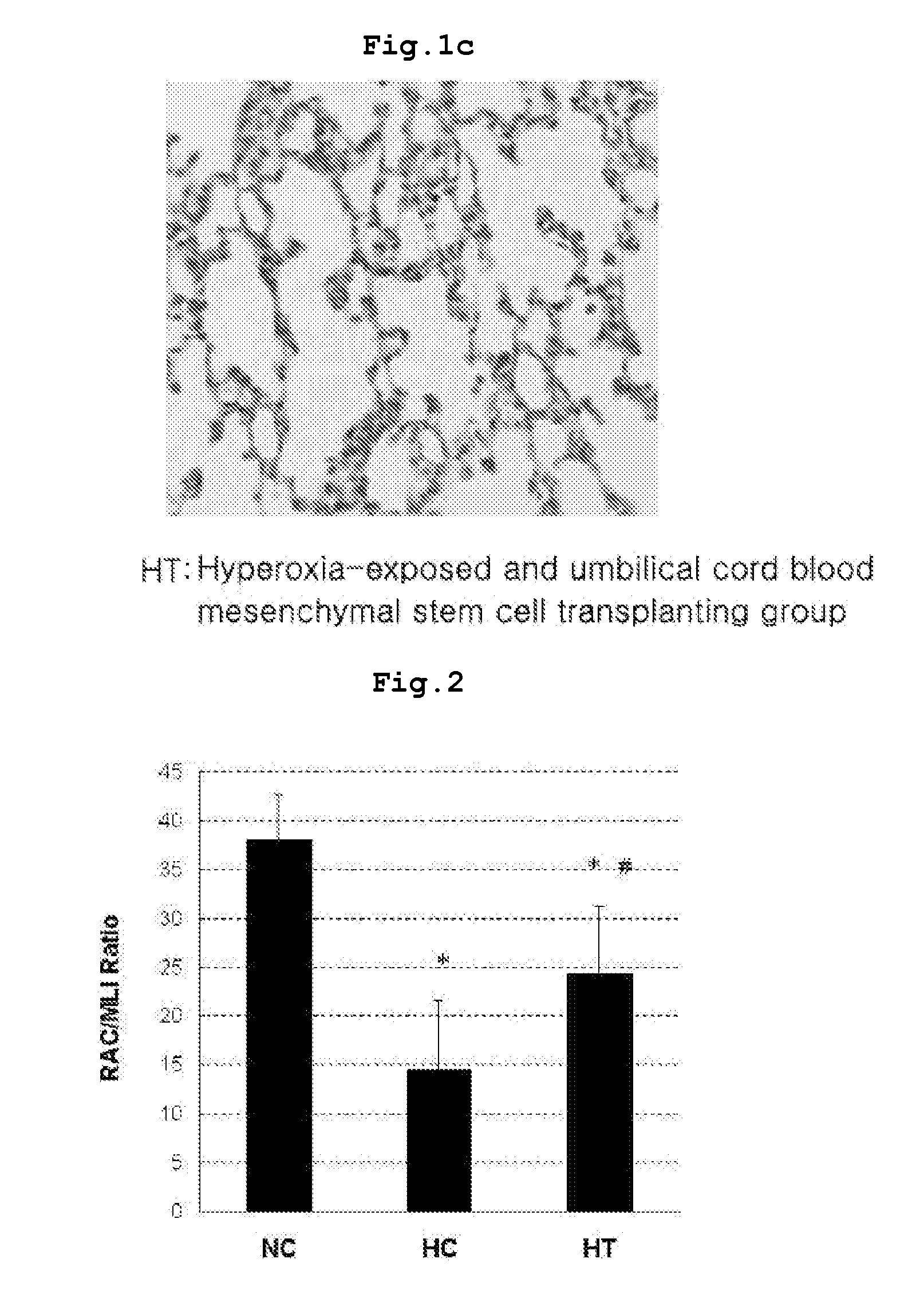
fibrosis with over-proliferated interstitial fibroblasts in the lung (see FIG. 1-FIG. 3). Besides, radial alveolar count (RAC) representing the number of alveoli was significantly decreased mean linear intercept (MLI) representing the size of alveoli was remarkably increased, and resultantly the ratio of RAC to MLI, an alveolar development index, was significantly reduced, compared with that in the
wild type normal rat.
 Login to View More
Login to View More  Login to View More
Login to View More