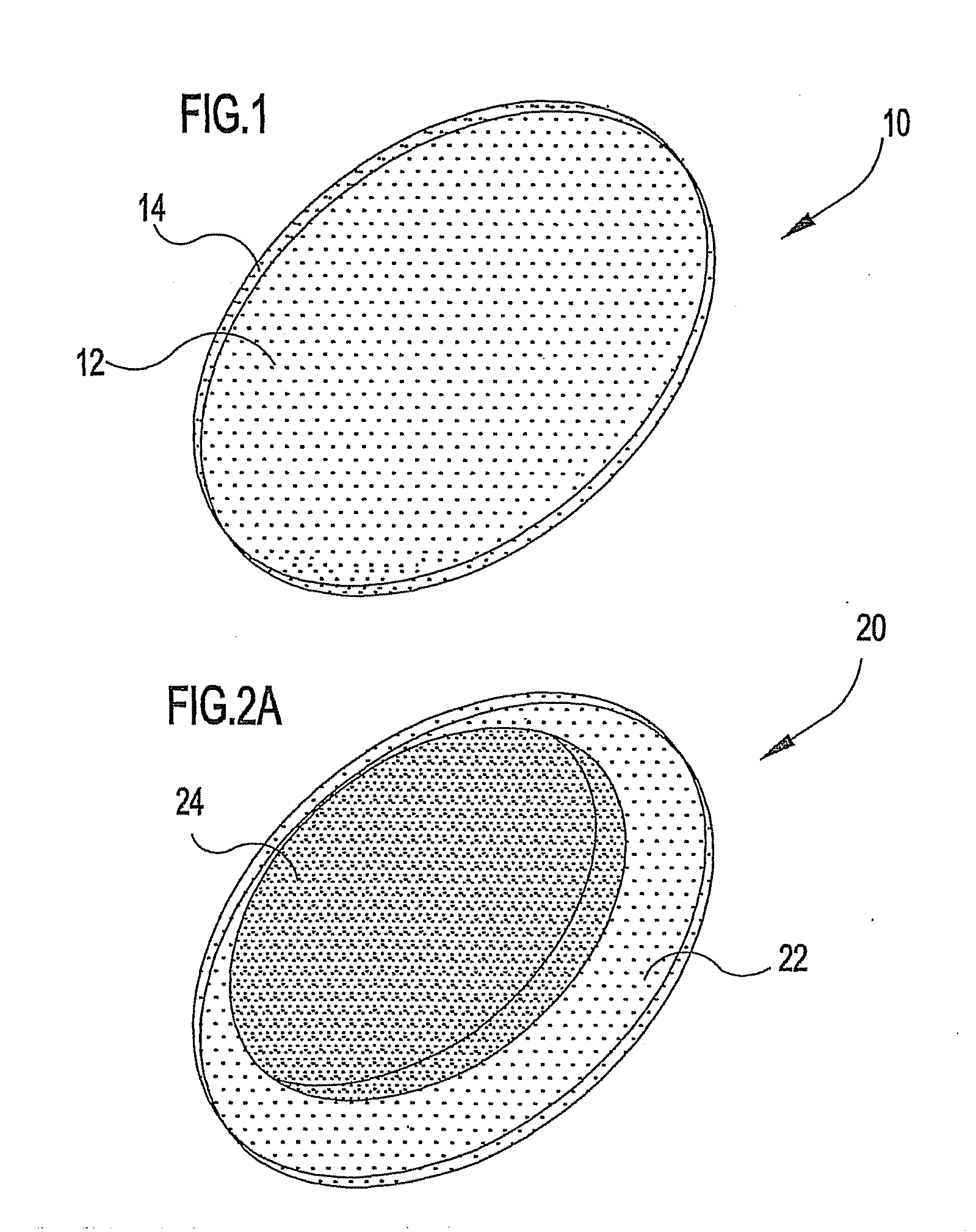
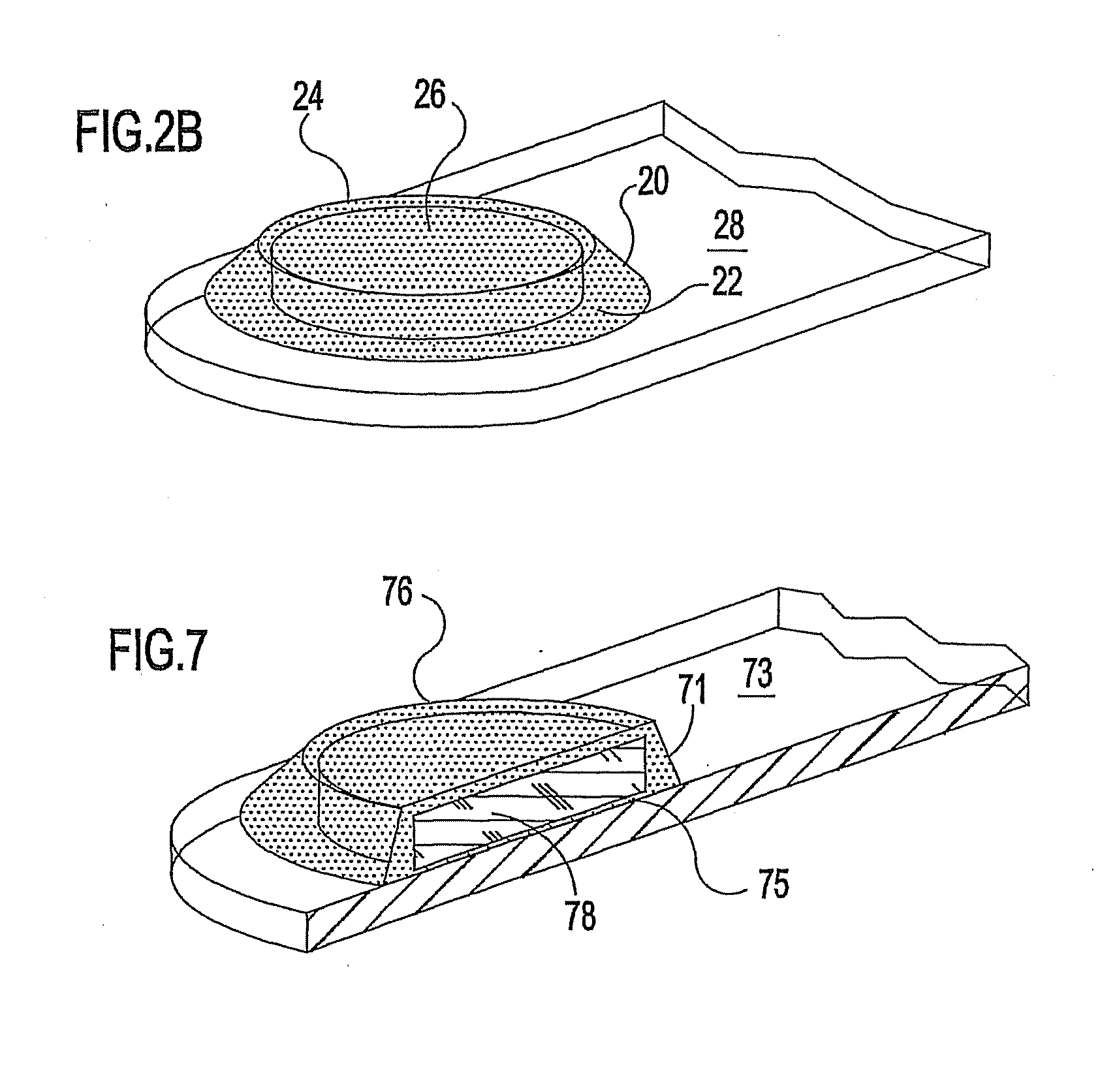
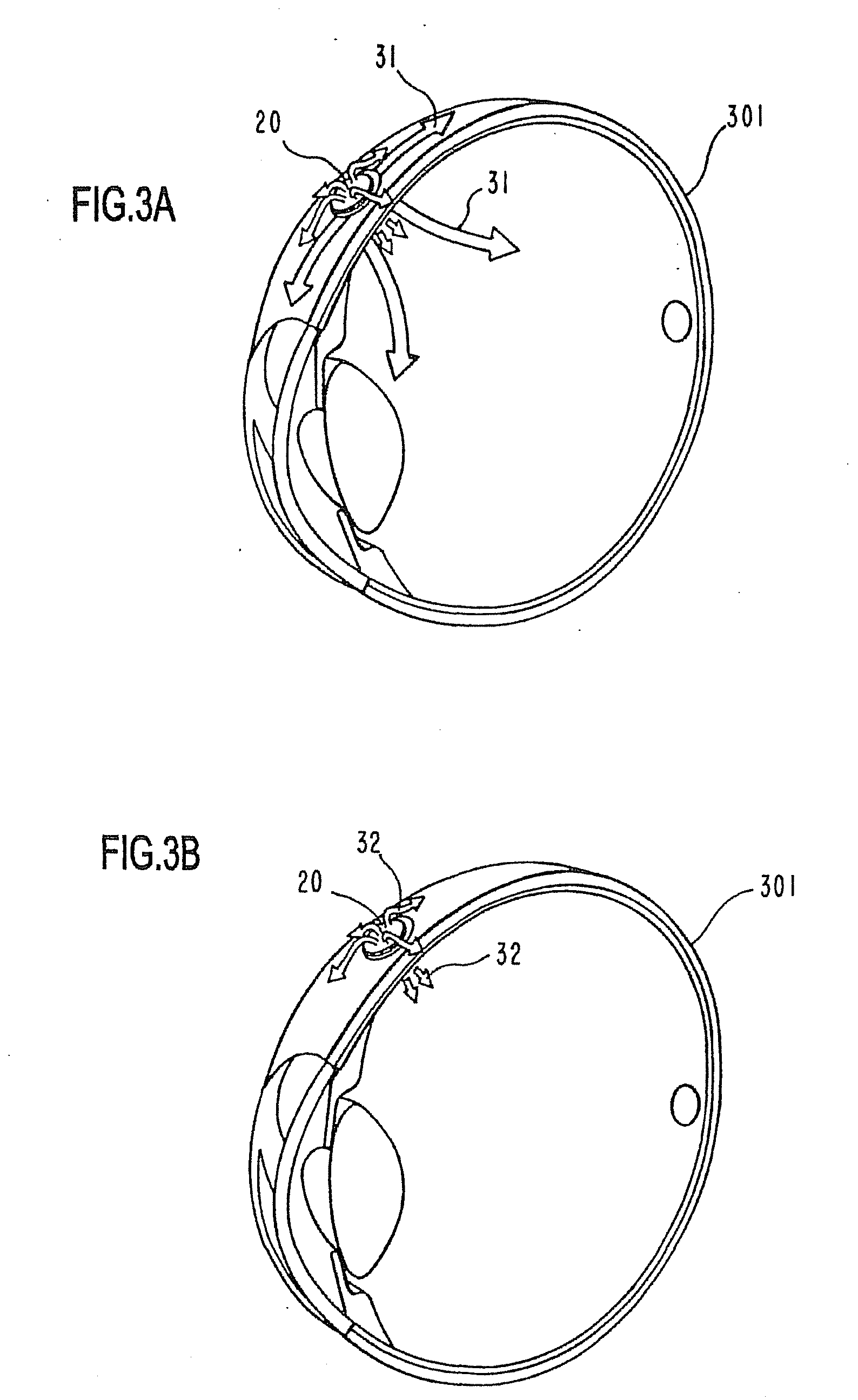
Ocular therapeutic agent delivery devices and methods for making and using such devices
a technology of ocular therapeutic agent and delivery device, which is applied in the field of controlled release of ocular implant device, can solve the problems of side effects, negligible actual absorption of drug in ocular tissues, and failure of oral therapies for the eye to provide sustained release of drug into the eye, etc., and achieves the effects of high release rate or loading dose, high release rate, and constant drug delivery ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0208] This example illustrates the preparation of a matrix, implant of the invention useful for subconjunctival implants.
[0209] 4.5 g of superhydrolyzed polyvinyl alcohol (Airvol 125, Air Products and Chemicals, Inc., Allentown, Pa., U.S.A.) was added to 30 ml of molecular biology grade water in an assay tube that was then tightly closed. The tightly closed assay tube was placed in a beaker of boiling water until the density becomes uniform (generally about 3-7 hrs). Since the assay tube was tightly closed, the contents could not evaporate. Water was periodically replaced in the beaker to keep the water height near the height of water in the assay tube. The assay tube was centrifuged for 1 minute at 1000-4000 rpm to degass the mixture. This formed a 15 wt % solution of superhydrolyzed polyvinyl alcohol.
[0210] Separate premixtures were prepared using each of cyclosporine A and 2ME2 as the therapeutic agent. Each therapeutic agent was separately premixed in a solution of hydroxypro...
example 2
[0213] This example illustrates the preparation of another matrix implant of the invention, which is useful as an intravitreal implant. Alternatively, this matrix implant can be used for an inlay used in combination with reservoir implants of the invention described elsewhere herein.
[0214] Preparation of 50% Superhydrolyzed PVA, 6% 2ME2, 0.05% HPMC Matrix Implant:
[0215] The polymer drug mixture was prepared in a 3 cc syringe (the tip sealed with a Luer lok and a HPLC septum). The plunger was removed. A drug emulsion was prepared by adding 63.8 mg 2ME2 and 0.5 mg hydroxypropyl methylcellulose (E4M, Dow Chemical) to 2 ml molecular grade biological water. The emulsion was mixed with a magnetic stirrer over night, and then it was added to 1 g superhydrolyzed PVA (Airvol 125; Air Products and Chemicals, Inc., Allentown, Pa., U.S.A.) in the syringe. The mixture was stirred until uniform and then placed into a water bath at about 100° C. for 60 minutes. The sample with the syringe was th...
example 3
[0216] A 1×1×2 mm matrix implant was prepared using poly(ethylene vinyl) acetate (EVA) in place of superhydrolyzed PVA in the subconjunctival implant.
[0217] Preparation of 30% EVA, 6% 2ME2, 0.05% HPMC Matrix Implants:
[0218] The polymer drug mixture was prepared in a 3 cc syringe (the tip sealed with a Luer lok and a HPLC septum). The plunger is removed. A drug emulsion is prepared by adding 38.3 mg 2ME2 and 0.3 mg HPMC (E4M, Dow Chemical) to 2 ml methylene chloride. The emulsion is mixed over night with a magnetic stirrer and then transferred to a 10 ml vial containing 0.6 g EVA (Elvax 40W, Dupont). The mixture was stirred with a magnetic stirrer until it becomes too viscous. The magnetic stirrer was then removed and the mixture was left overnight. The sample was centrifuged as needed to degas the specimen. The specimen was poured onto a glass plate and permitted to dry for 48 hours under vacuum. The implants were then cut to size using a razor blade, e.g., multiple 1×1×2 mm slabs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com