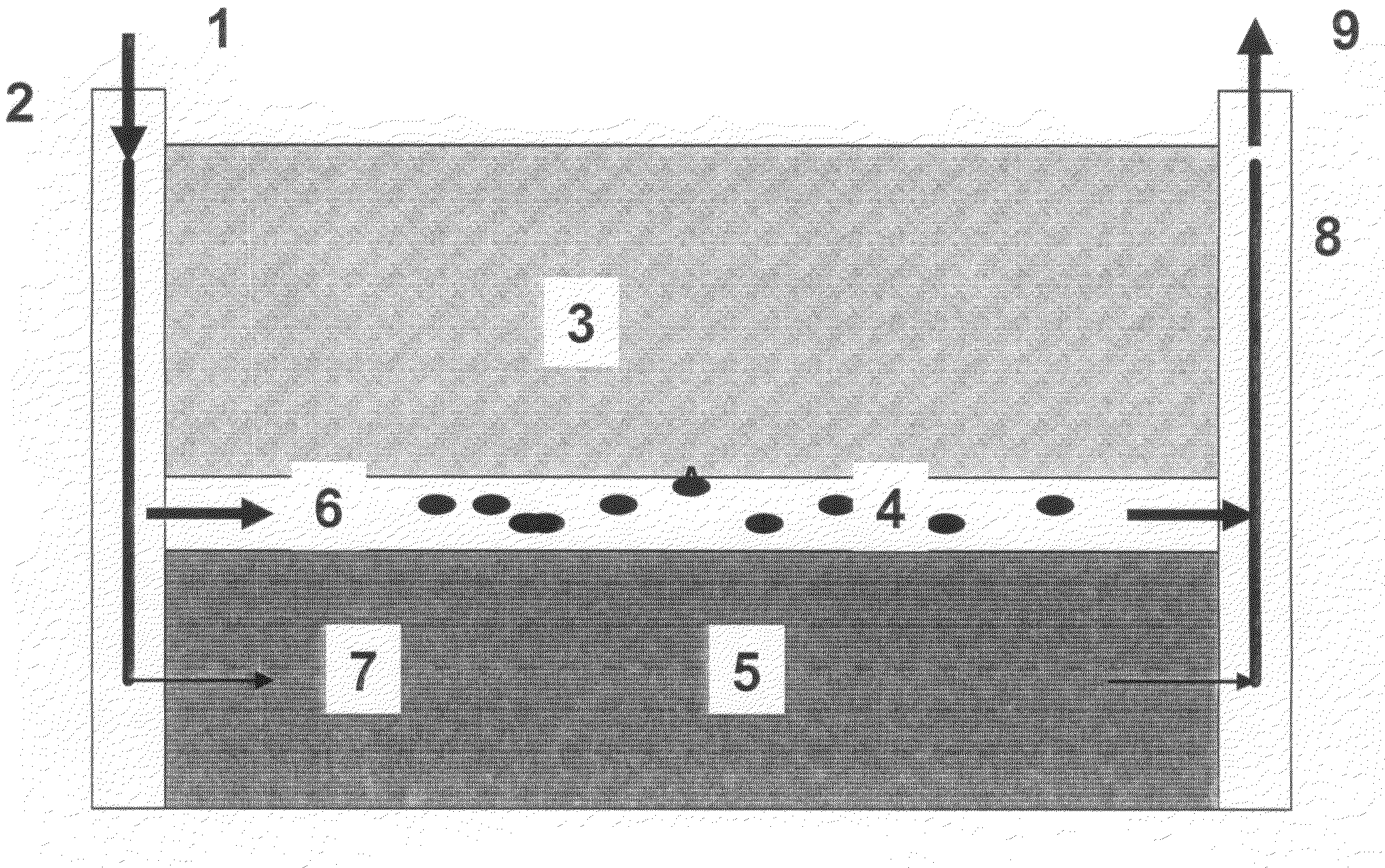
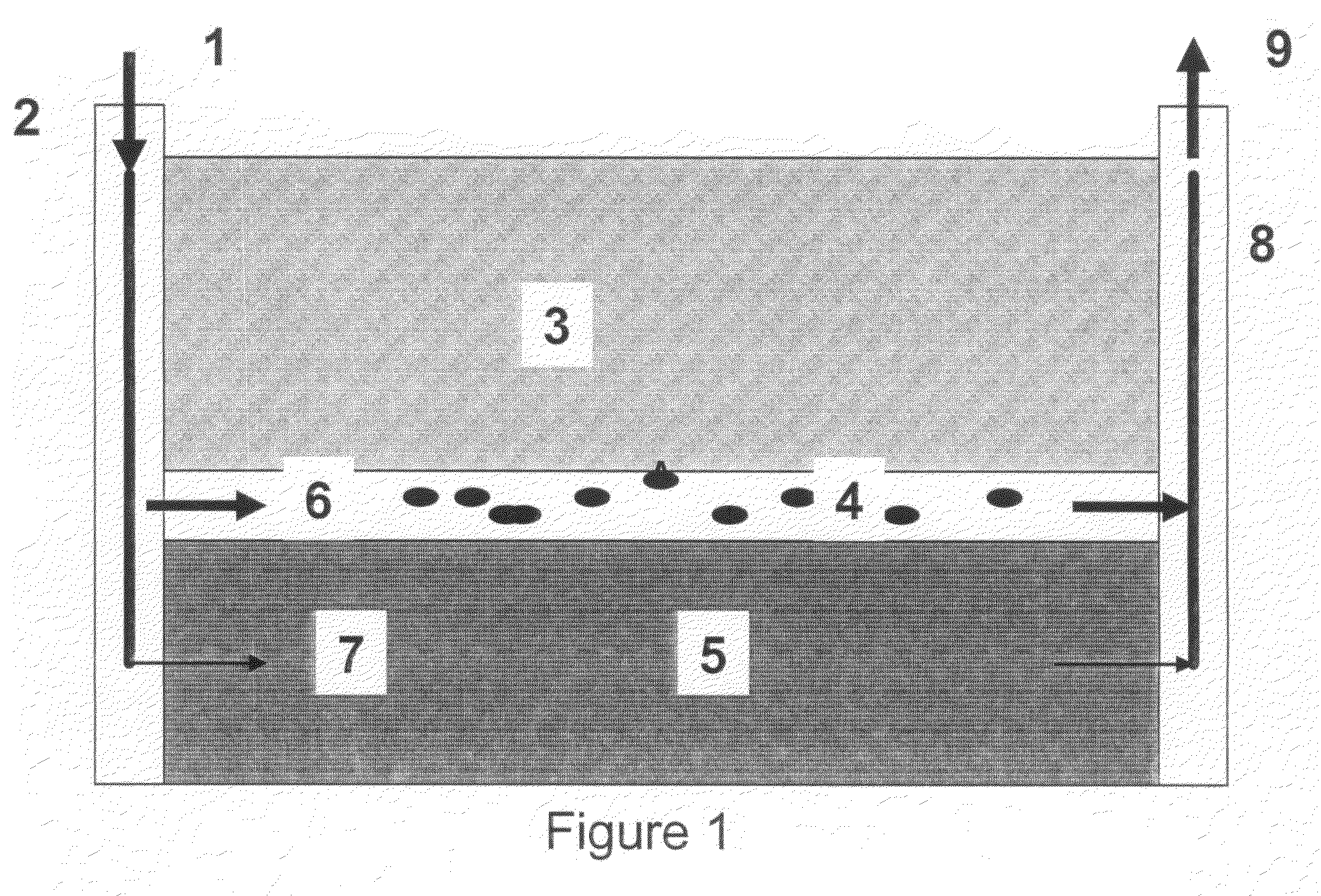
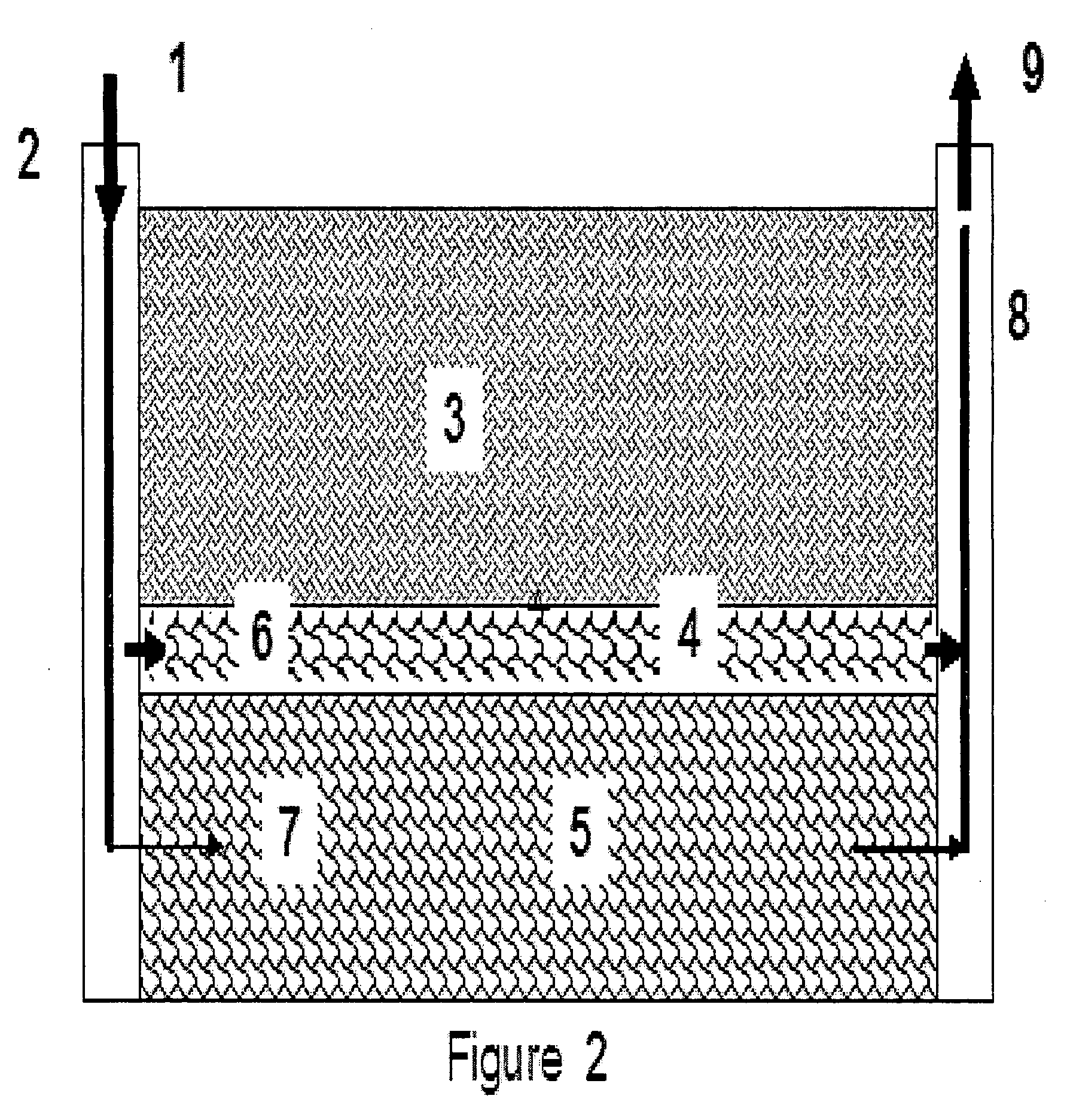
Preformed particle gel for conformance control in an oil reservoir
a technology of conformance control and oil reservoir, which is applied in the direction of fluid removal, chemistry apparatus and processes, and well accessories, etc., can solve the problems of non-uniform displacement of oil within the reservoir, inability to homogenize the geologic properties of many reservoirs from which oil and gas are produced, and inability to achieve uniform water injection. achieve the effect of small initial siz
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Sample 27
[0035]In the present example, a single aqueous phase was prepared by adding 8.25 g acrylamide, 21.75 g sodium salt of 2-acrylamido-2-methylpropane sulfonic acid, 0.386 g polyethylene glycol 200 diacrylate, and 0.0004 g methylene bisacrylamide to 30.6 g deionized water with then mixing. At an ambient temperature of 15-30° C., an initiator mixture of 400 μl 5% sodium bromate and 400 μl 5% sodium bisulfite was added slowly to the solution with strong mixing. Within about 5 minutes, the reaction of polymerization took place with heat released, resulting in a fine gel.
example 2
Preparation of Sample 31
[0036]In this example, the procedure of example 1 was repeated except that 6.10 g polyethylene glycol 200 diacrylate and no methylene bisacrylamide were added to the formula. All other components and reaction conditions remained the same.
example 3
[0037]Comparison of swelling behavior of Sample 27 versus Sample 31 demonstrates the controllability the swell time and extent with our composition.
[0038]Sample 27 and Sample 31 suspensions were both prepared at a concentration of 1 wt % in distilled water and had the pH adjusted to be between 8 to 9. A portion of each suspension was aged at 40 degree C. and at 60 degree C. for 2 days. The results are shown below:
Temperature(Centigrade)Sample 27Sample 3140fluidfluid60stiff gelfluid
[0039]These results demonstrate that the composition of the Sample 27 is suitable for an application where the controlled monomer is designed to decompose within 2 days at 60 degree C. Furthermore, the fact that the Sample 27 remains in a fluid state over the same aging time at 40 degree C. indicate that for Sample 27 the mechanism for the loss of effectiveness of the controlled monomer, thereby causing particle expansion and a gel to form is related to its exposure to a greater extreme in temperature to 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com