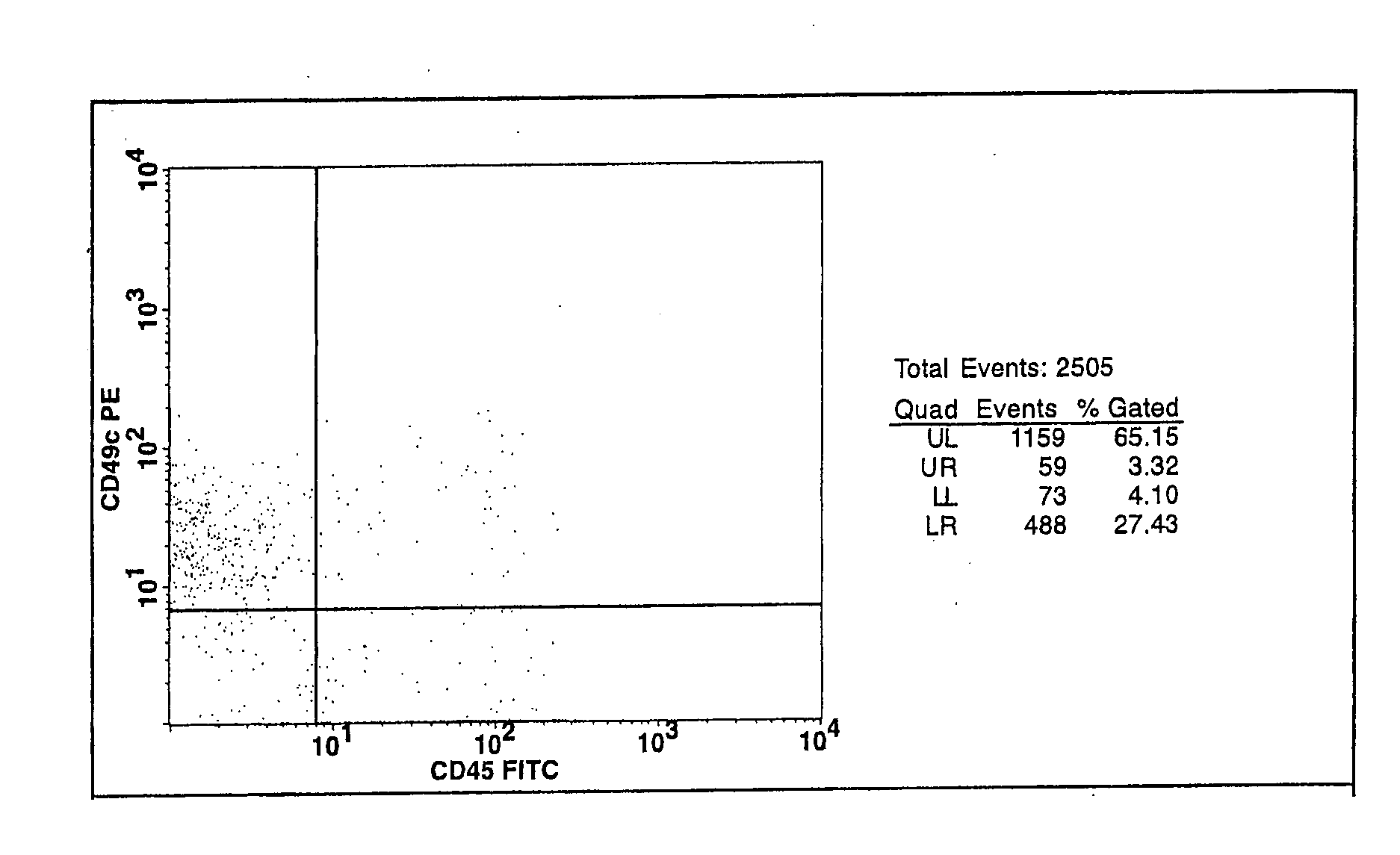
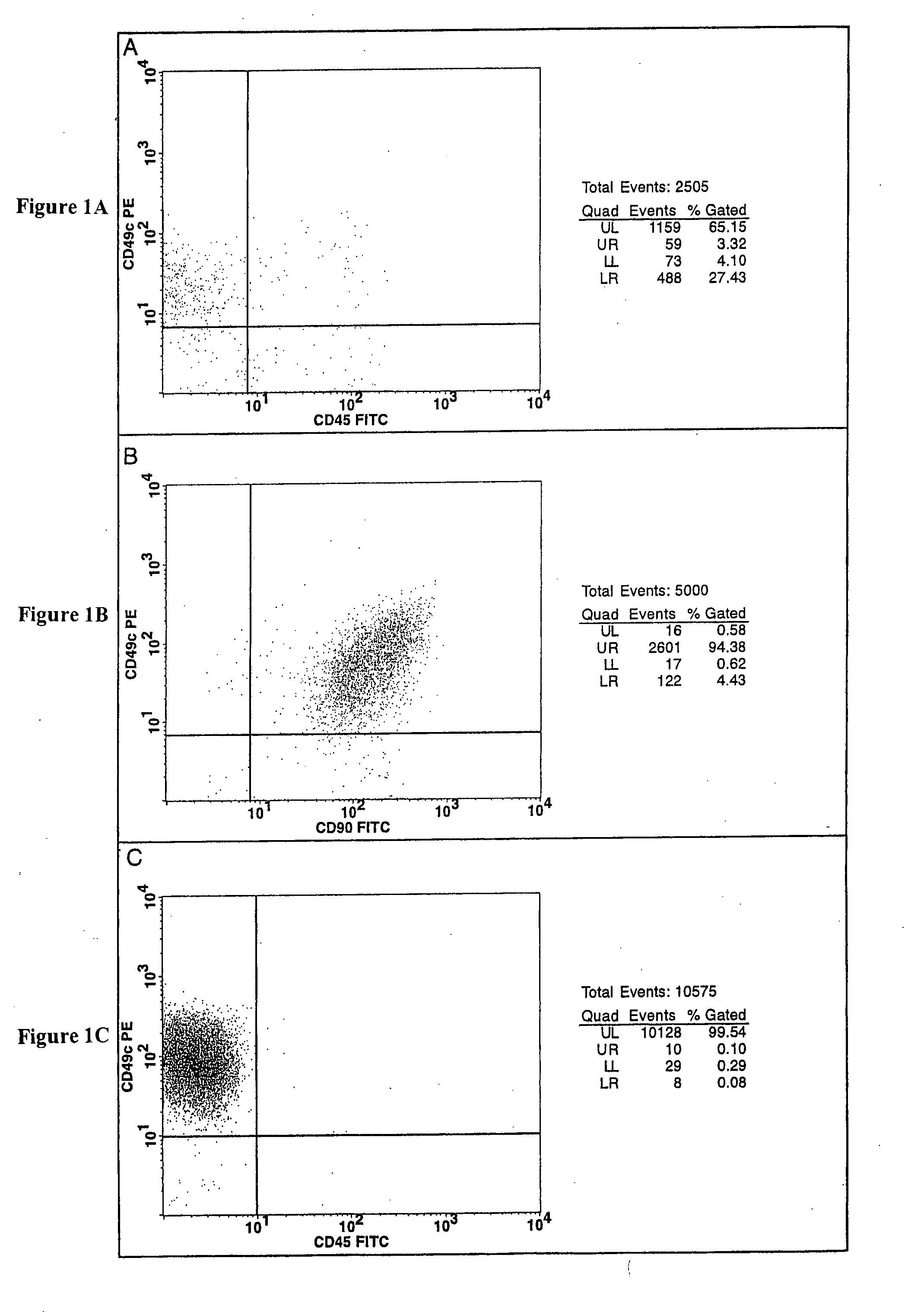
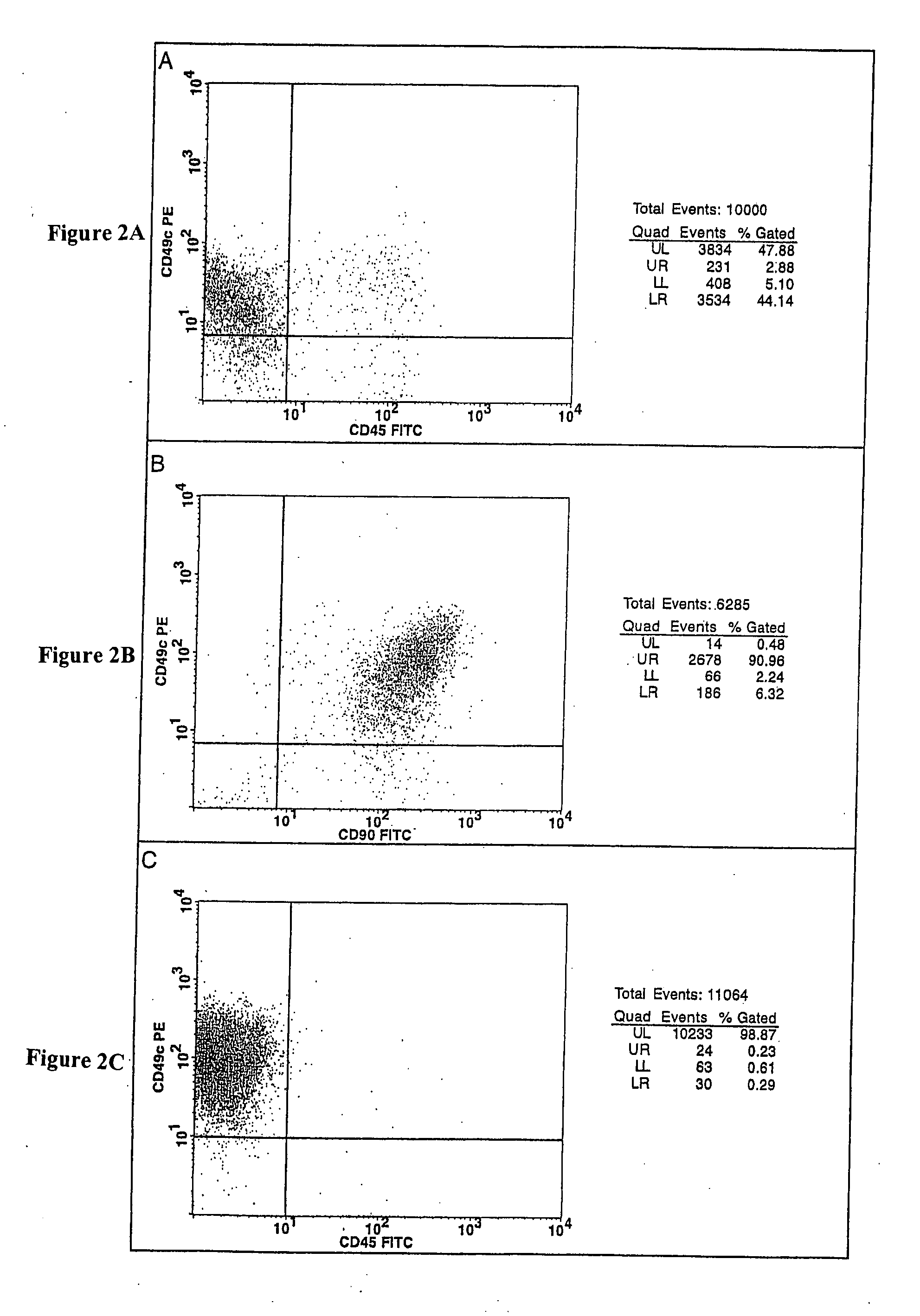
Cell populations which co-express CD49c and CD90
a technology of cd49c and cd90, which is applied in the field of cell populations which co-express cd49c and cd90, can solve the problems of cord) and peripheral nervous system adversely affecting humans, and achieve the effects of reducing or repairing loss, improving quality of life and life expectancy, and facilitating repair
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of a Adherent as Colony Forming Units of Cells or “CFUs” From Bone Marrow Aspirates Following Red Blood Cell Lysis
[0116] Bone marrow cells were aspirated from the iliac crest of healthy adult human volunteers. The red blood cell component of the aspirate was lysed by mixing the aspirate with an ammonium chloride buffer consisting of 155 mM ammonium chloride, 10 mM potassium bicarbonate and 0.1 mM EDTA (ethylenediaminetetraacetic acid), pH 7.2, at a 1:20 ratio of marrow aspirate to buffer. The resulting cell suspension was vortexed for 2 seconds, incubated for 2 minutes at ambient temperature and then centrifuged (10 minute at 500×g). The resulting mononuclear cell pellet was resuspended in complete medium and centrifuged (10 minutes at 500×g). Complete media is Minimal Essential Medium-alpha (Gibco BRL, Rockville, Md.) supplemented with 4 mM glutamine and 10% sera-lot selected fetal bovine serum (FBS, Gibco BRL, Rockville, Md.). The cell pellet was then re-suspended in th...
example 2
Isolation of a Adherent CFUs From Bone Marrow Aspirates Following Density Separation
[0121] Bone marrow cells were aspirated from the iliac crest of healthy adult human volunteers. The bone marrow aspirate was diluted with calcium and magnesium free phosphate buffered saline (PBS) to achieve a mononuclear cell concentration of 7×106 cells / mL and overlaid onto an equal volume of Histopaque® 1.119 (Sigma, St. Louis, Mo.) and centrifuged (30 min at 700×g). The resulting mononuclear cell fraction was transferred to a clean centrifuge tube containing PBS and centrifuged (10 minutes at 500×g). The cell pellet was re-suspended in PBS and centrifuged (10 minutes at 500×g). The supernatant was aspirated from the cell pellet and the cells re-suspended in complete media.
[0122] The number of viable cells in the resulting cell suspension was determined by trypan blue-exclusion. The cell suspension was then seeded in tissue culture-treated T75 flasks at a density of 50,000 cells / cm2 and incubate...
example 3
Production of Primary and Master Cell Banks from CFUs
[0124] After 7-10 days in culture, the CFUs generated using the methods described in Example 1 were removed from the T75 flasks with a 0.25% trypsin / 1 mM EDTA solution (Life Technologies). After 10 minutes at 37° C., the trypsin was inactivated with 10 mL of complete medium. The cells were washed once with HBSS and re-suspended in Glycerol Cell Freezing Medium® (Sigma Chemical Co.). Aliquots (referred to herein as “the Primary Cell Bank”) of the suspension consisting of 4.0×105 cells / vial were cooled with liquid nitrogen vapor at 1° C. / minute using a CryoMed (Forma) controlled rate freezer and the stored in a Cryo Plus liquid nitrogen storage tank (Forma).
[0125] An aliquot of cells was removed from the Primary Cell Bank and cultured at a density of 30 cells / cm2 in 500 cm2 tissue culture-treated plates (Corning) in complete medium and incubated at 37° C. in an atmosphere consisting of 5% carbon dioxide, 5% oxygen, and 90% nitroge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| doubling time | aaaaa | aaaaa |
| doubling time | aaaaa | aaaaa |
| doubling time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com