Transition metal-substituted hydrotalcite catalyst for removing nitrogen oxides from the exhaust gas of diesel engine by storage-reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
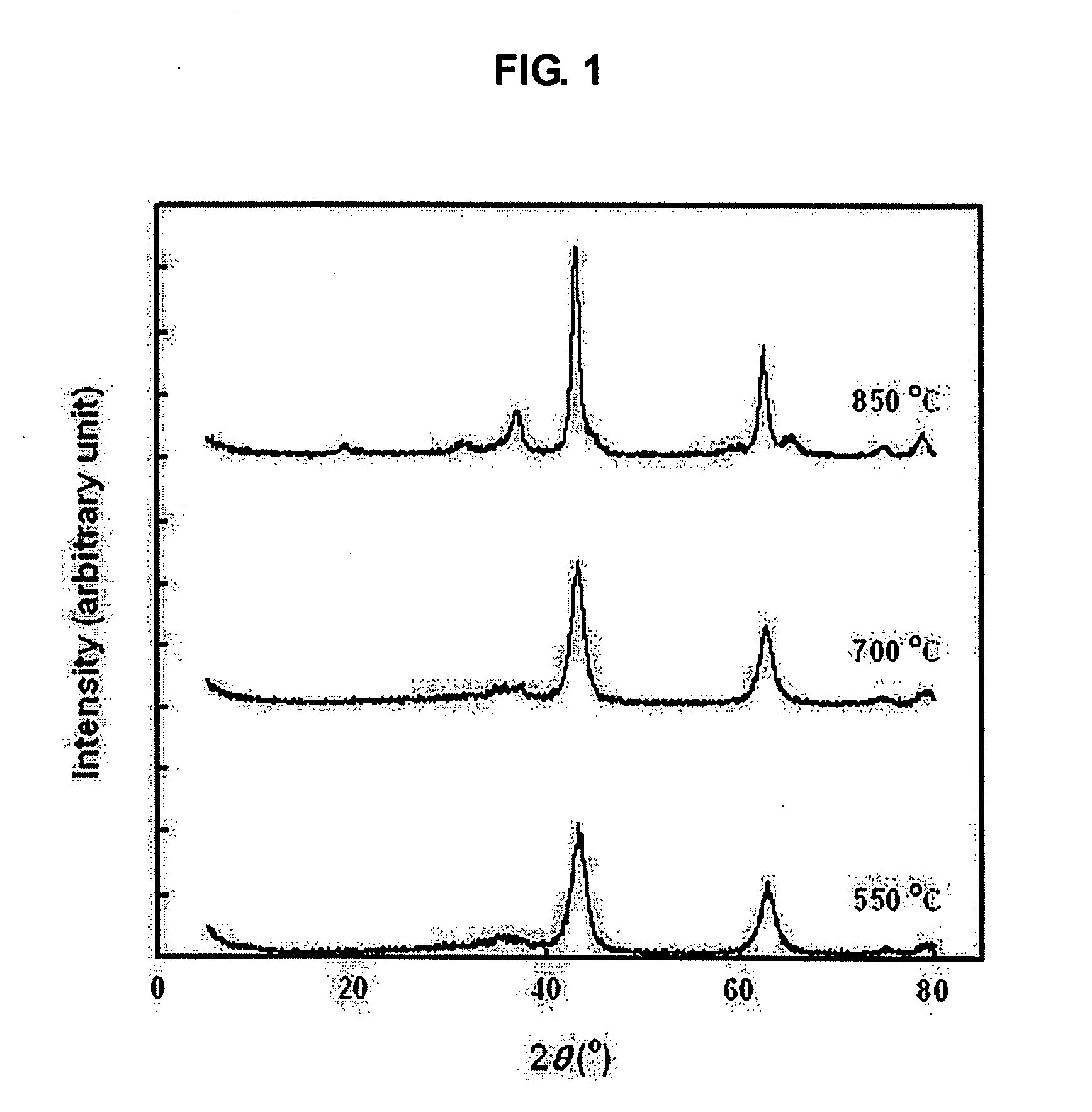
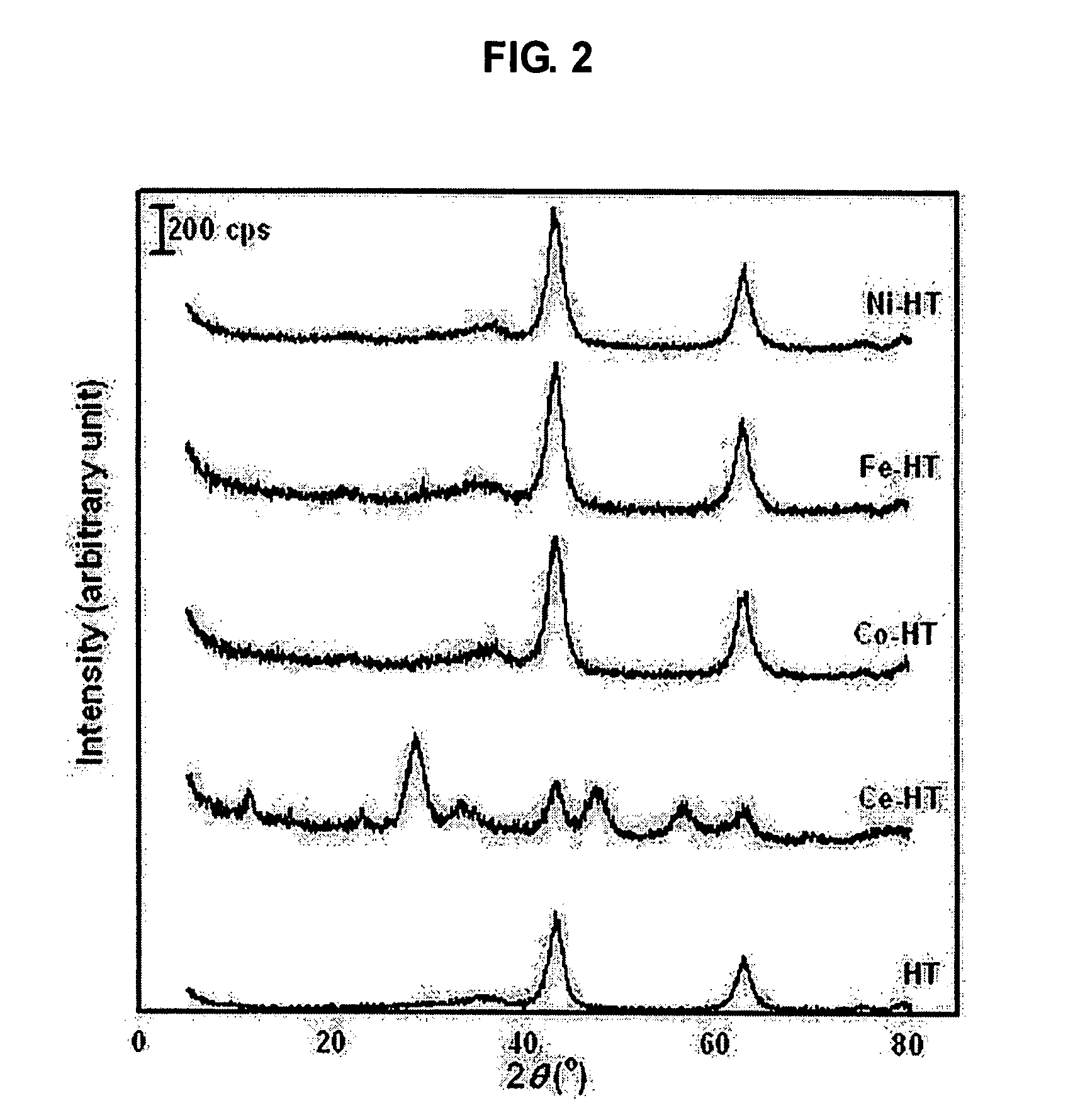
[0023] A hydrotalcite catalyst for storing and removing nitrogen dioxide from the exhaust gas of diesel engines was manufactured using a hydrothermal method. 68.5 g of aluminum nitrate and 145 g of magnesium nitrate were put in 500 ml of water and then sufficiently stirred to be completely dissolved in water. Further, 65 g of sodium hydroxide and 53.5 g of sodium carbonate were also dissolved in 500 ml of water. These two solutions were mixed, stirred at room temperature for 24 hours, and then aged. Subsequently, the aged solution was put in a high-pressure reactor, heated to a temperature of 100° C., and then reacted at this temperature for 4 hours. The reaction products were washed while solid products were filtrated therefrom, and were then dried at a temperature of 100° C. The dried reaction products were baked in an electrical furnace at a temperature of 550˜850° C., thereby preparing hydrotalcite (HT). The amount of magnesium and aluminum, which are constituent elements of the...
experimental example 1
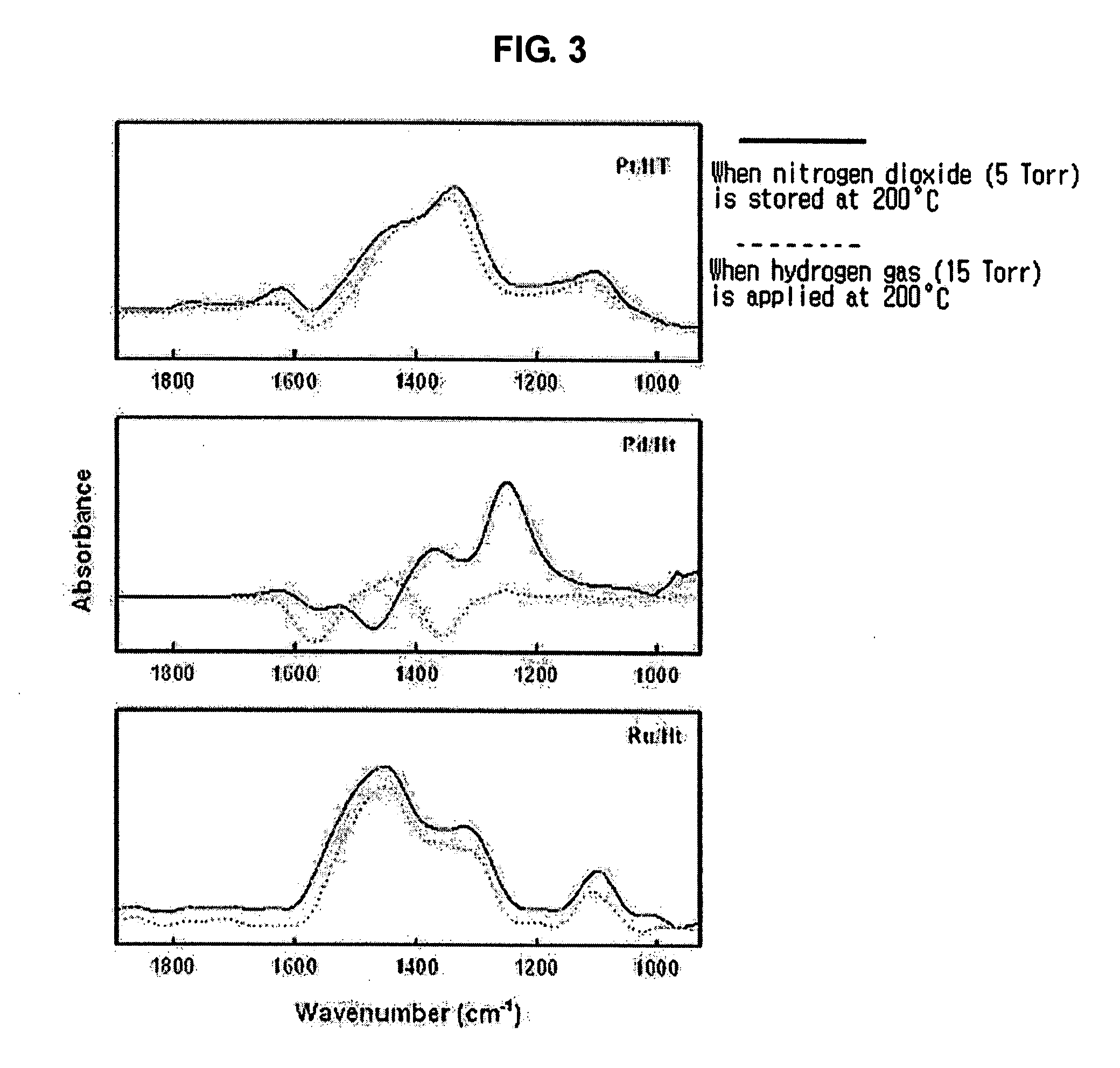
[0025] The amount of stored nitrogen dioxide was measured using a weight-type adsorption apparatus provided with a quartz spring balance. 0.05 g of a hydrotalcite catalyst was put in a quartz basket and was subjected to exhaust at a temperature of 300° C. for 1 hour. In consideration of the temperature of the exhaust gas of diesel automobiles, 20 Torr of nitrogen oxide gas was added thereto at a temperature of 200° C. When the catalyst was exposed to the nitrogen oxide gas, nitrogen oxide is stored in the catalyst, and thus the weight of the catalyst was increased. When there is no increase in the weight of the catalyst, the mass increase of the catalyst in the presence of the nitrogen oxide gas is defined as “adsorption amount”, and the mass increase measured after the catalyst was subjected to the exhaust is defined as “storage amount”.
[0026] The adsorption amount and storage amount of nitrogen dioxide of hydrotalcite catalysts baked at various temperatures are given in Table 1. ...
example 2
[0027] The hydrotalcite, which was prepared using the method describe in Example 1 and was then baked at a temperature of 550° C. for 6 hours, was hydrothermally treated at a temperature of 550˜850° C. while dripping nitrogen including 10 wt % of water thereinto. Even if the hydrotalcite was hydrothermally treated, the X-ray diffraction peaks of the hydrotalcite were not considerably changed, but the characteristic peaks of MgAl2O4, having a spinel structure, were slightly exhibited. Compared to the crystallinity calculated based on a sample hydrothermally heated at temperature of 550° C., the crystallinity of a sample hydrothermally heated at temperature of 850° C. was 93.4%, which is high. The crystallinity of the hydrothermally-heated hydrotalcite and the adsorption amount and storage amount of nitrogen dioxide, measured using the method described in Experimental Example 1, are given in Table 2.
TABLE 2Adsorption amount and storage amount of nitrogendioxide of hydrothermally-tre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com