Stable liquid interferon formulations
a liquid formulation and interferon technology, applied in the direction of drug compositions, peptide/protein ingredients, immunological disorders, etc., can solve the problems of lack of interferon stability in solutions and other products, especially in purified form, and limited utility of proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Assay Methods
[0079] Several well-characterized methods are used to determine the physico-chemical properties of the interferon-beta in our liquid formulations and these methods may be used to monitor properties of other interferons as well.
[0080] The presence / absence of insoluble aggregate is monitored by measuring the absorbance at 320 nm and transmittance at 580 nm. The concentration of soluble protein is determined by either measurement of absorbance at 278-280 nm (with an extinction coefficient of 1.5) or by reverse-phase high performance liquid chromatography (HPLC) using known concentrations of interferon-beta spiked in the formulation buffer as standards. The liquid formulation samples are centrifuged prior to assay. The soluble aggregate percentage is determined by separating aggregates from interferon-beta monomer by size exclusion chromatography on a TSK-Gel® G2000SWXL column (Toso Haas, Montgomeryville, Pa.). The peak areas monitored at 280 nm are used to calculated the...
example 2
Choice of Buffer System
[0086] We prepared three sets of buffers containing between nine and 10 different components for each set. Set I contains a series of sodium phosphate and / or 100 mM sodium chloride solutions between pH 4.0 and 7.2. Set II contains an additional series of sodium citrate buffers between pH 4.0 and 7.0. Set III contains a series of sodium succinate, sodium acetate and sodium carbonate buffer solutions, all combined with 100 mM sodium chloride, having pH values ranging from 4.0 to 7.2. Two other solutions replaced the sodium chloride with 50 mM sodium sulfate at a pH of 4.0 and 7.2.
[0087] Thawed, bulk interferon-beta is dialyzed into different buffers overnight at 2-8 degrees C. with at least two buffer exchanges, then sterile filtered prior to use. Protein concentrations are determined by absorbance at 278 nm (with extinction coefficient of 1.5 mg−1 ml·cm−1) and all samples contained 140 ug / ml or 150 ug / ml interferon-beta. Samples are filtered and split into fo...
example 3
The Effect of Cavitation
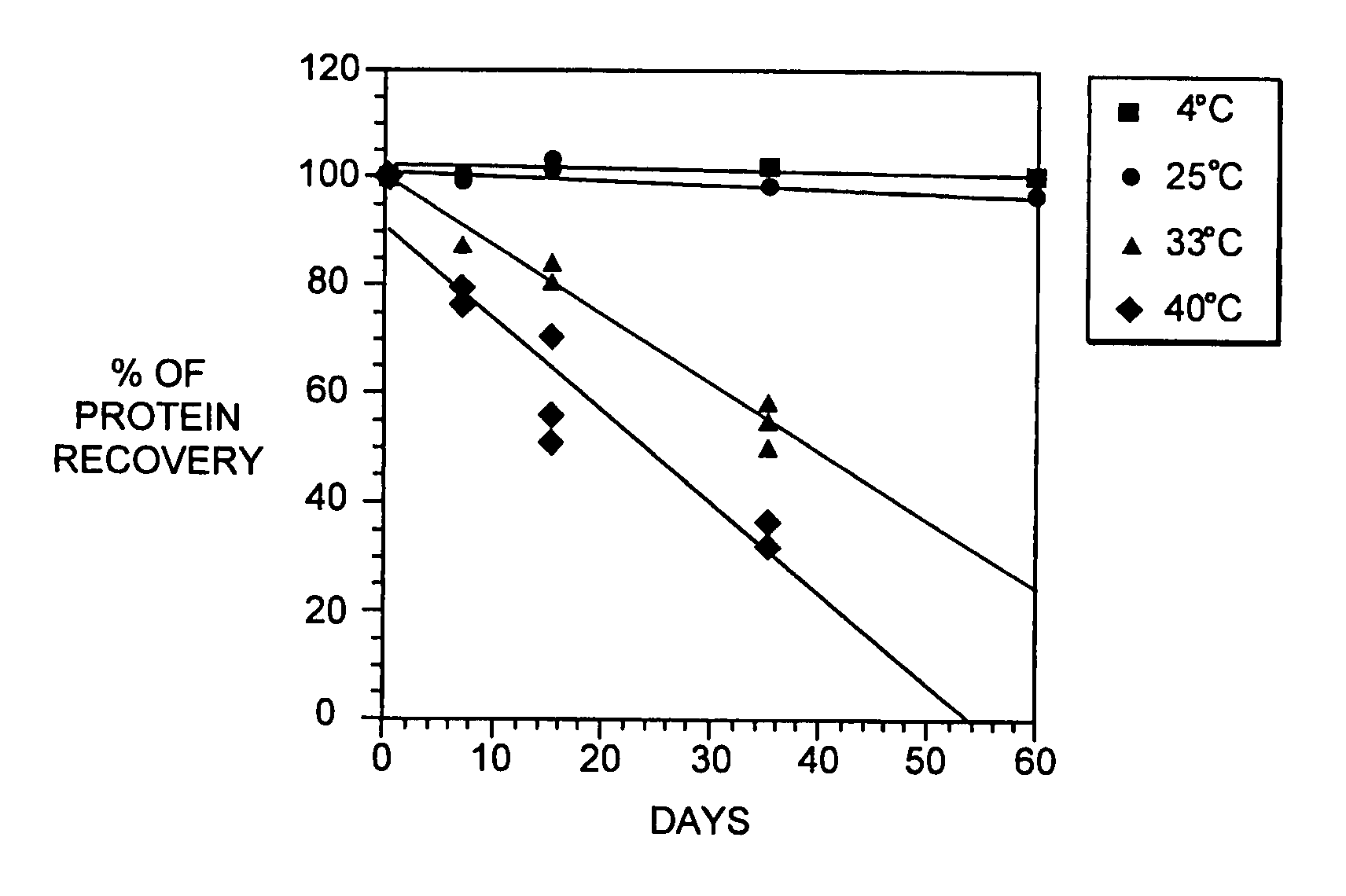
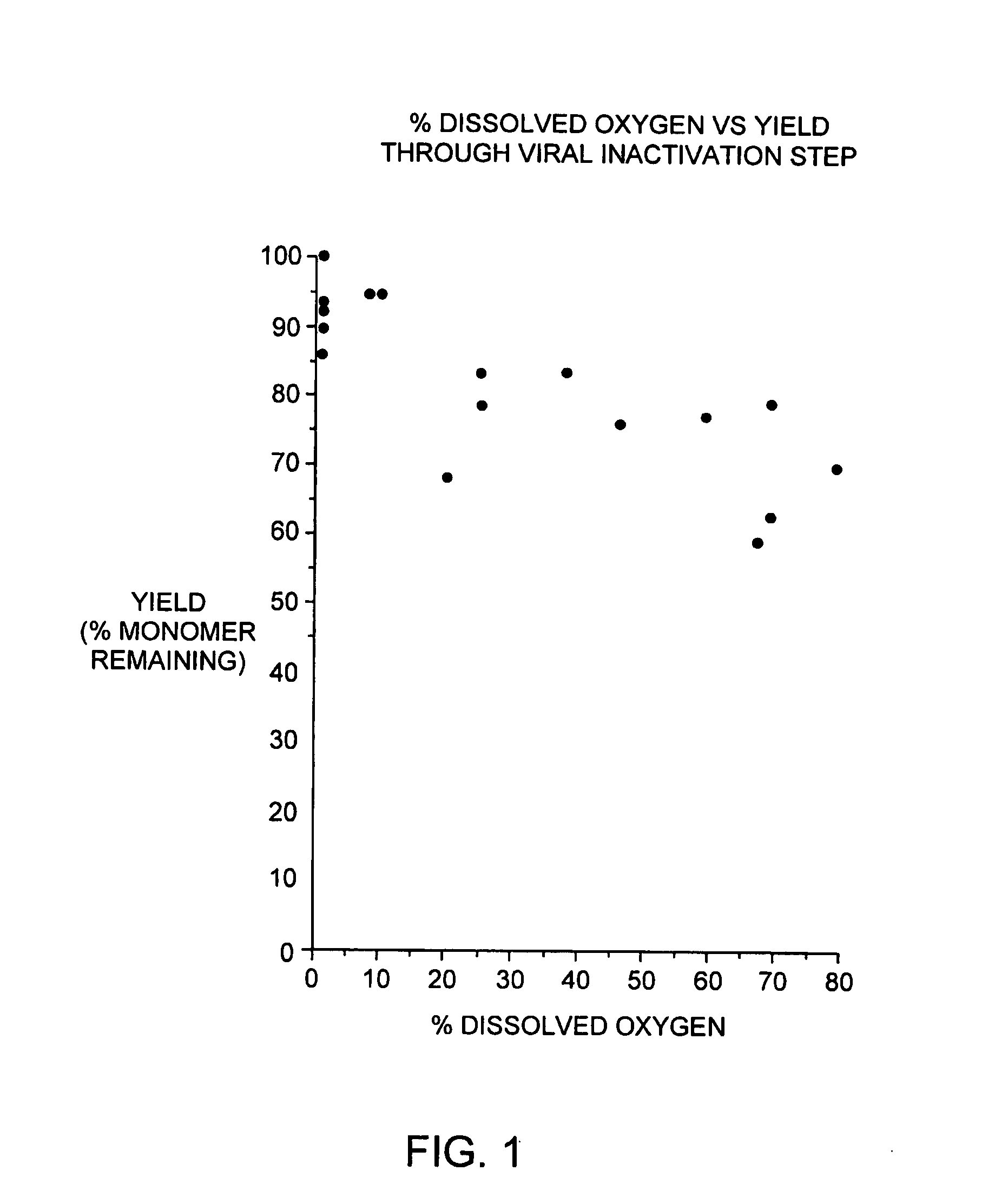
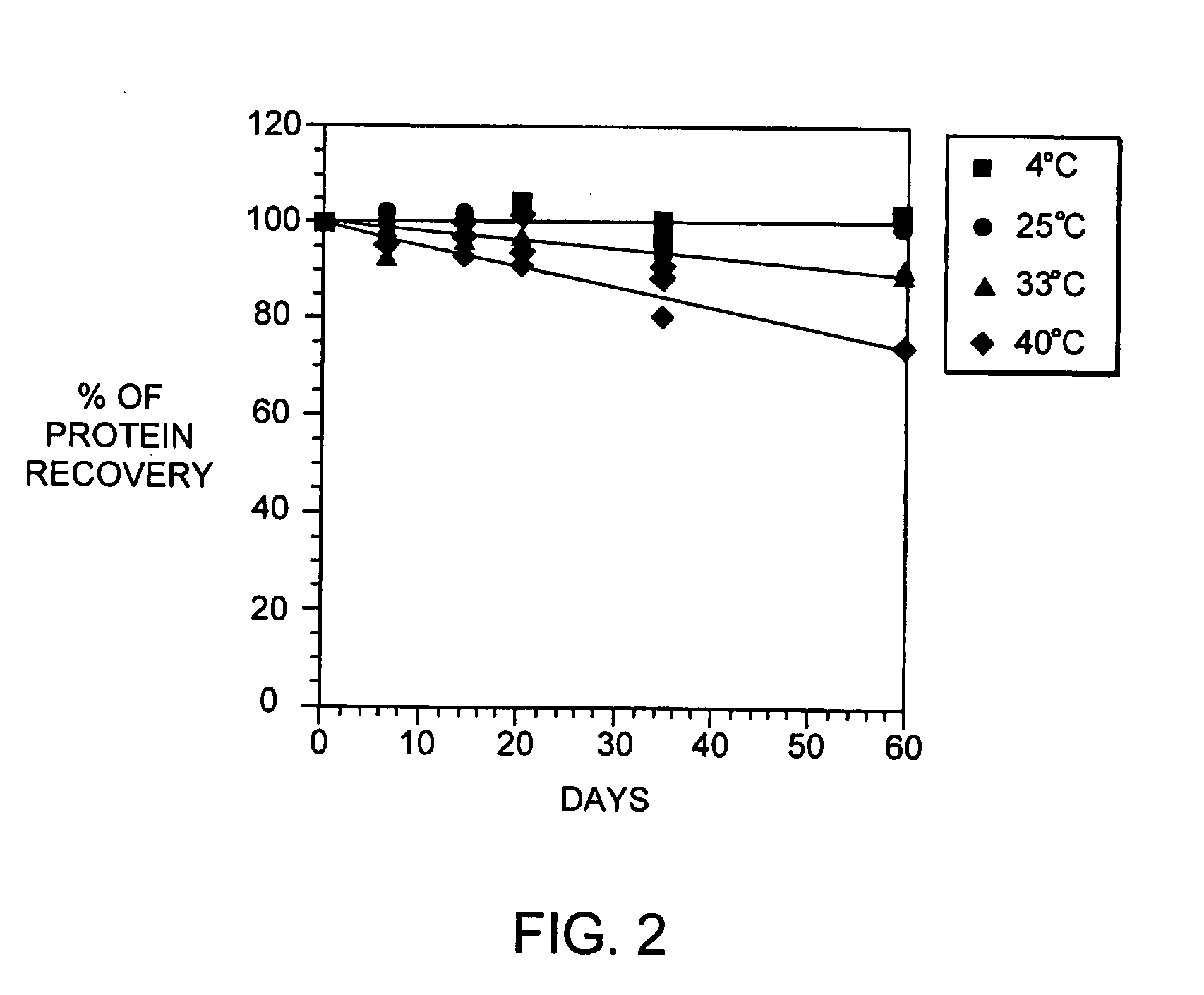
[0089] During our pH screening experiments described in Example 2, we discovered that the head space of the storage tubes appears to be critical for loss of protein of some of the samples. With 1.5 ml of the samples in 2.2 ml volume tubes, no loss of protein was observed. On the contrary, 1.2 ml of sample produced significant increase in aggregates. This is consistent with our observations that formation of aggregated interferon-beta during the viral inactivation step of the purification process is dependent on the level of dissolved oxygen during this step.
[0090] In brief, the viral inactivation step involves adjusting the pH of the chelating Sepharose eluate (see Section C) from 7.85±0.25 to between 2.5 to 3.5 with 15% phosphoric acid, holding the acidified eluate for 120-135 minutes, and then readjusting the pH to 6.7±0.7 with 0.5 N sodium hydroxide. All steps are performed at 2-8 degrees C. We designed a study to determine if a relationship exists betwe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com