Composition of entomopathogenic fungus and method of production and application for insect control
a technology of entomopathogenic fungus and entomopathogenic bacteria, which is applied in the direction of biocide, plant/algae/fungi/lichens ingredients, plant cultivation, etc., can solve the problems of poor application practicability, poor shelf life or lyophilization of room temperature fungus, and formulations too expensive for general use, etc., to achieve excellent shelf life, low moisture level, and comparable or even higher levels and rates of infectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Microsclerotia
[0022]In this example we have evaluated different liquid culture nutritional environments and measured biomass accumulation and blastospore and microsclerotia yields. The desiccation tolerance of microsclerotia was measured by evaluating their ability to germinate vegetatively and / or sporogenically upon rehydration.
[0023]Three strains of Metarhizium anisopliae var. anisopliae (Metchnikoff) Sorokin were used in this study: a commercial strain, F52 (ATCC 90448, (Earth Biosciences, now Novozyme Biologicals, Salem, Va., reisolated from Tetanops myopaeformis larvae), MA1200 (ATCC 62176, passaged through T. myopaeformis larvae), and TM109 (ARSEF5520 reisolated from T. myopaeformis larvae). All isolates were stored at −80° C. at USDA ARS NPARL and at USDA ARS NCAUR. Stock cultures of each strain of M. anisopliae were grown as single spore isolates on potato dextrose agar (PDA) for three weeks at room temperatures. The sporulated plate was cut into 1 mm2 agar plu...
example 2
Assessment of Fungal Outgrowth and Sporulation From Microsclerotia on Different Soils
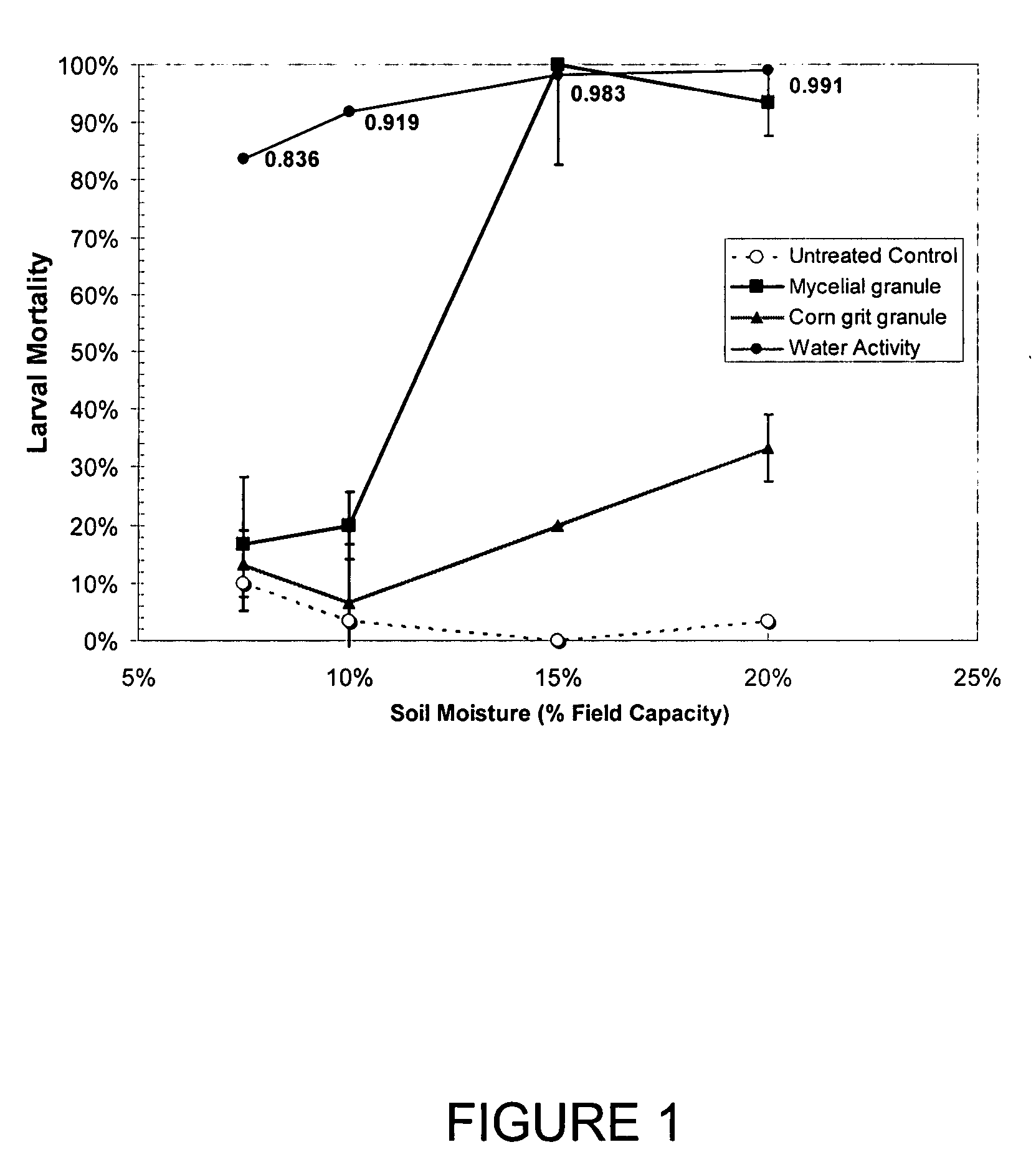
[0031]The fungal outgrowth and sporulation of M. anisopliae strain MA1200 from microsclerotial granules produced from Medium 4, 5, and 6 were evaluated on moist soil plates. The granules were prepared as described earlier, sieved to a 0.6-1.7 mm particle size, and stored in sealed plastic bags at 5-7> C. for 9 months prior to use. A clay soil from a sugarbeet field in Sidney Mo., a clay loam soil collected from a sugarbeet field near St. Thomas, N.D., and a sandy-loam soil from Torrington, Wyo. were separately air dried to a moisture content less than 2%, pulverized and sieved through as 20-mesh (U.S.) sieve to a uniform particle size range. Soil texture was determined by standard methods (Sheldrick and Wang, 1993, Particle size distribution. In, Soil Sampling and Methods of Analysis, M. R. Carter, Ed. Can. Soc. Soil Science, Lewis Publishers, Boca Raton, Fla., pp. 499-512). All soils tested were no...
example 3
Relative Efficacy of Microsclerotial Granules Produced by the Six Media in Example 1
[0037]The relative biological efficacy of the microsclerotial granules produced by strain F52 in all six media was evaluated using soil-based bioassays with larval sugarbeet root maggots (SBRM). Granules (20 / 30 mesh) of F52 from all six media were incorporated into a dry, sieved, non-sterile clay soil used earlier at the rate of 14 mg granules / 60 g soil. Two separate production batches of granules were evaluated. The granules had been stored in sealed plastic bags at 5-9° C. for 7 months prior to use. The soils were moistened with reverse osmosis water to an end point of 15% Field Capacity (previously determined) and the water potentials determined with an AQUALAB moisture meter (Decagon Products, Pullman, Wash.). Resulting soil moistures were 0.982-0.983 Aw (−2.32 to −2.47 MPa matric potential), which moistures were sufficient for fungal outgrowth and sporulation. Permanent Wilting Point for most pl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com