Medicinal composition, food or drink having effect of enhancing sympathetic nervous activity
a technology of sympathetic nervous activity and composition, applied in the field of medicinal composition, can solve the problems of affecting the function of the body, and affecting the effect of the body, so as to achieve the effect of enhancing sympathetic nervous activity, and not causing a rise in blood pressur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
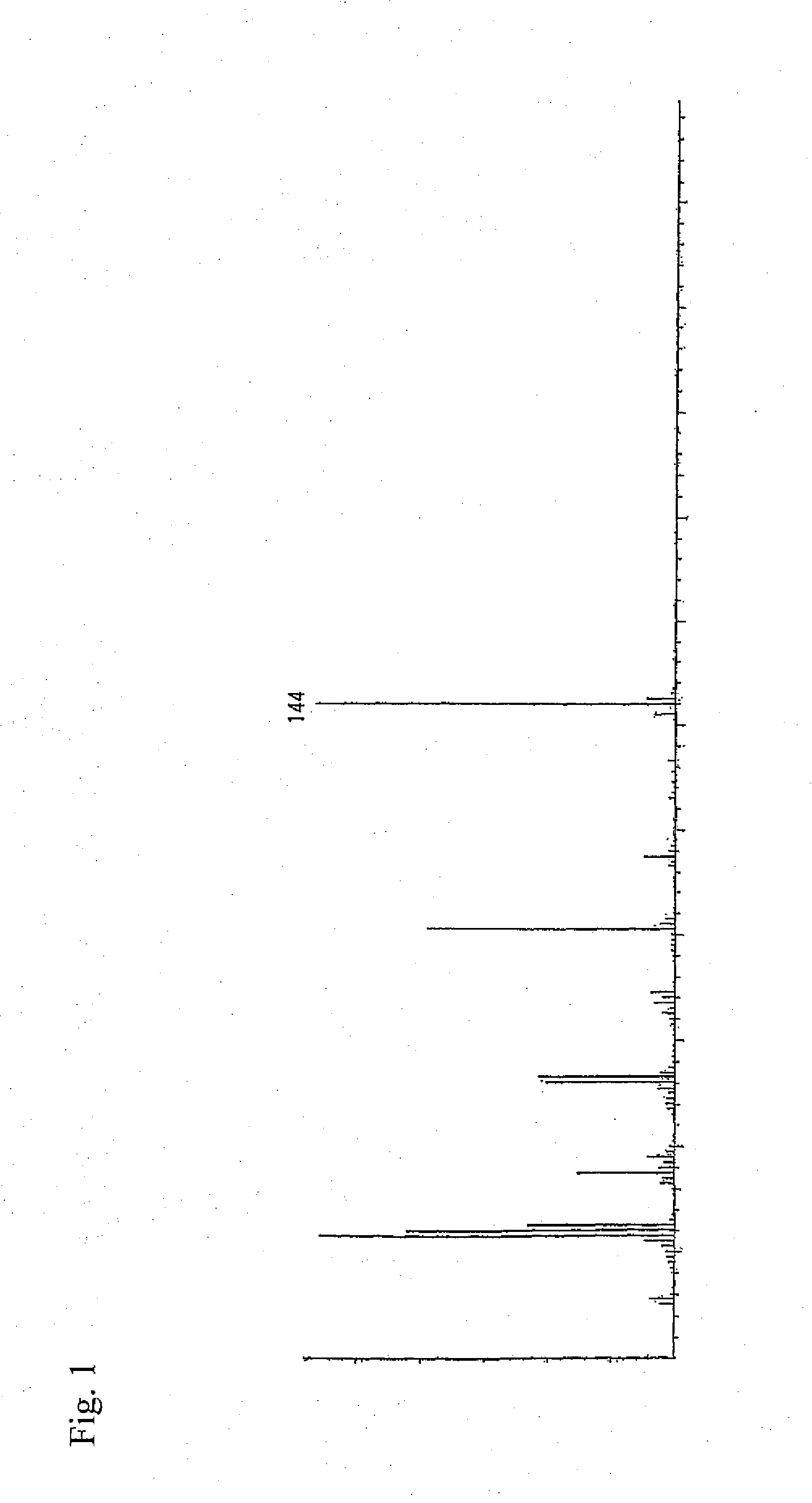
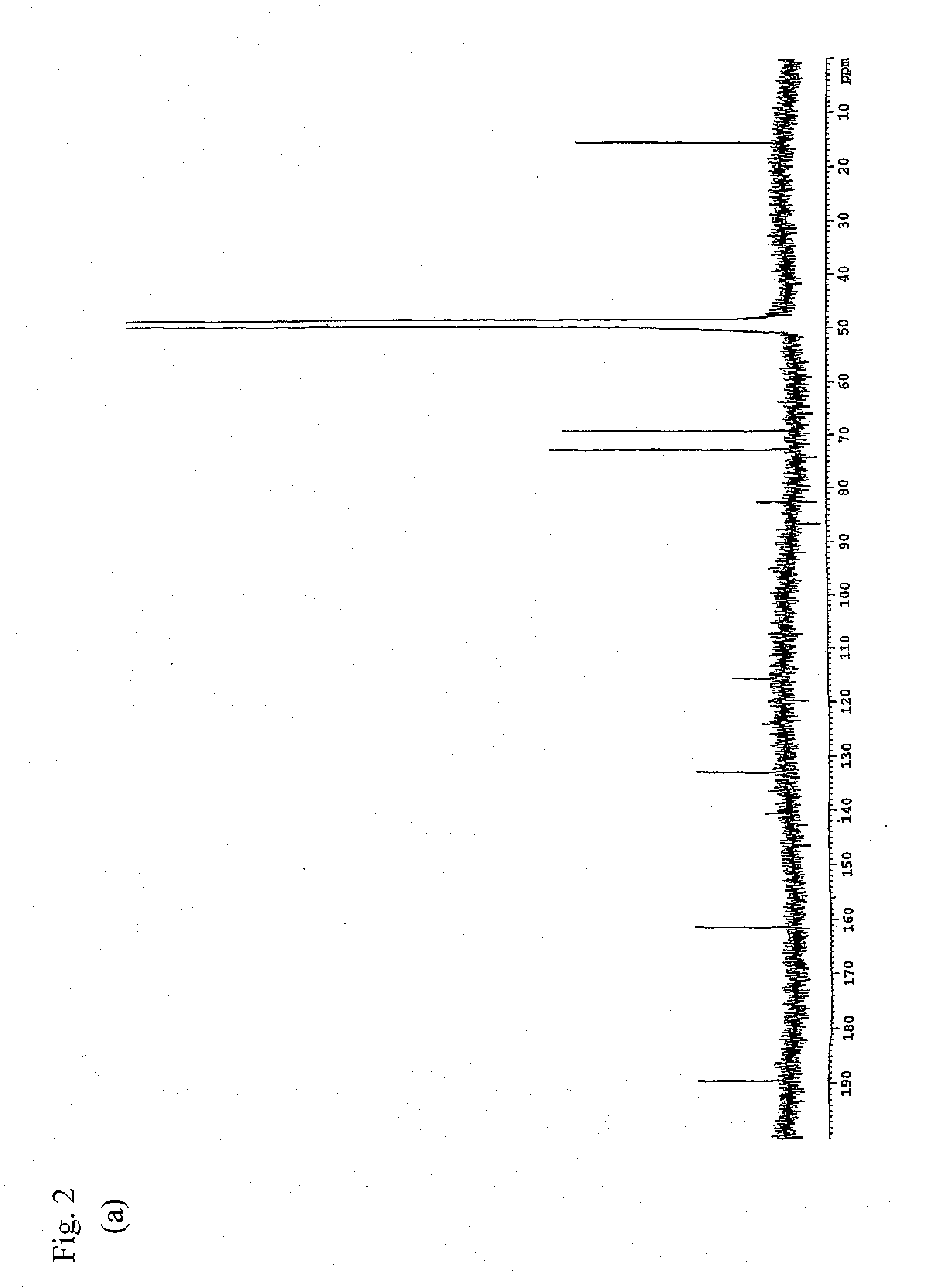
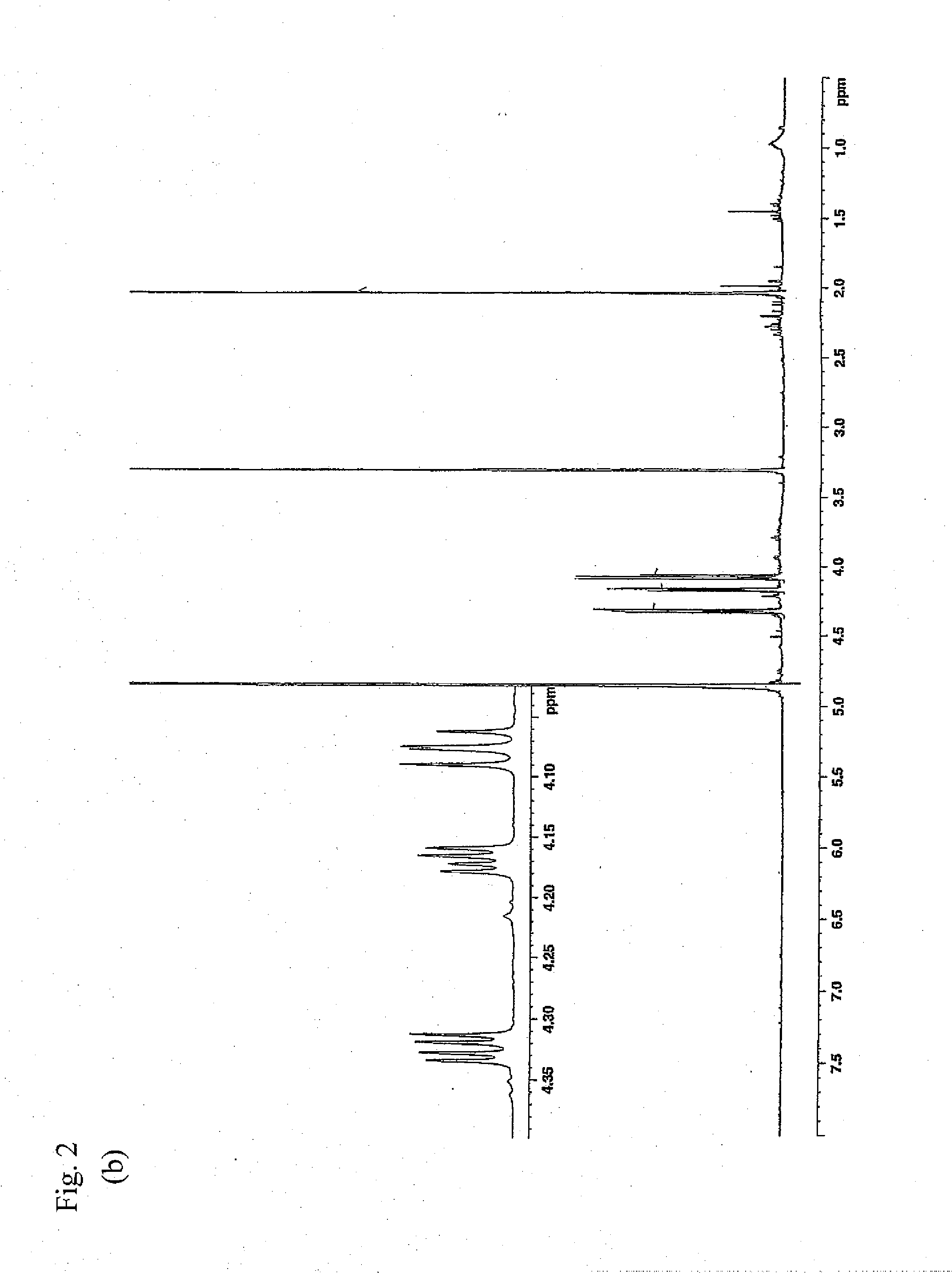
Preparation of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (Purification from Lactic Acid Bacterium Culture Supernatant)
[0092]As the lactic acid bacterium culture supernatant, use was made of a freeze-dried culture supernatant obtained by culturing Lactobacillus plantarum SAM 2446 strain (FERM ABP-10438) in MRS medium. A sterilized MRS medium was inoculated with Lactobacillus plantarum SAM 2446 strain (FERM ABP-10438) which was then statically cultured therein at 37° C. for 1 day. Next, the cells were removed by centrifugation to give a culture supernatant. By use of the culture supernatant thus obtained, a freeze dried product was prepared by a conventional method and employed as the starting material. First, the lactic acid bacterium culture supernatant was ultra-filtered using Ultrafiltration Membranes YM10 (manufactured by Millipore) to give a low molecular fraction passing through the membranes. Next, the obtained fraction was chromatographically fractionated through Deve...
example 2
Evaluation of the effects of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one on Rat Kidney Sympathetic Nervous Activity, Blood Pressure and Stomach Vagal (Parasympathetic) Nervous Activity
[0093]In these experiments, use was made of male Mister rats weighing about 300 g which had been fed in a thermostatic chamber (24° C.) with a light-dark cycle of 12-hour intervals (under illumination from 8:00 to 20:00) for 1 week or longer. A single rat was employed for each nervous experiment. The animals were fed with a feed (Oriental Yeast, MF) and water ad libitum. The autonomic nervous activity were examined by fasting the rats for 3 hours on the day of the experiment and then subjecting to an abdominal surgery under urethane-anesthesia at the intermediate point of the light period. Then, the effects of the transduodenal administration of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one obtained in Example 1 on the kidney sympathetic nervous activity, stomach vagal (parasympathetic) nervo...
example 3
Evaluation of the Effects of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one on Rat Interscapular Brown Adipose Tissue Sympathetic Nervous Activity and Epididymal White Adipose Tissue Sympathetic Nervous Activity
[0095]The procedure of Example 2 was followed and thus the effects of the transduodenal administration (10 μg) of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one obtained in Example 1 on the rat epididymal white adipose tissue sympathetic nervous activity and the interscapular brown adipose tissue sympathetic nervous activity were examined in accordance with the methods reported in the documents cited in Example 2. A single rat was employed for each nervous experiment.
[0096]As a result, both of the interscapular brown adipose tissue sympathetic nervous activity (FIG. 6) and the epididymal white adipose tissue sympathetic nervous activity (FIG. 7) were enhanced.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com