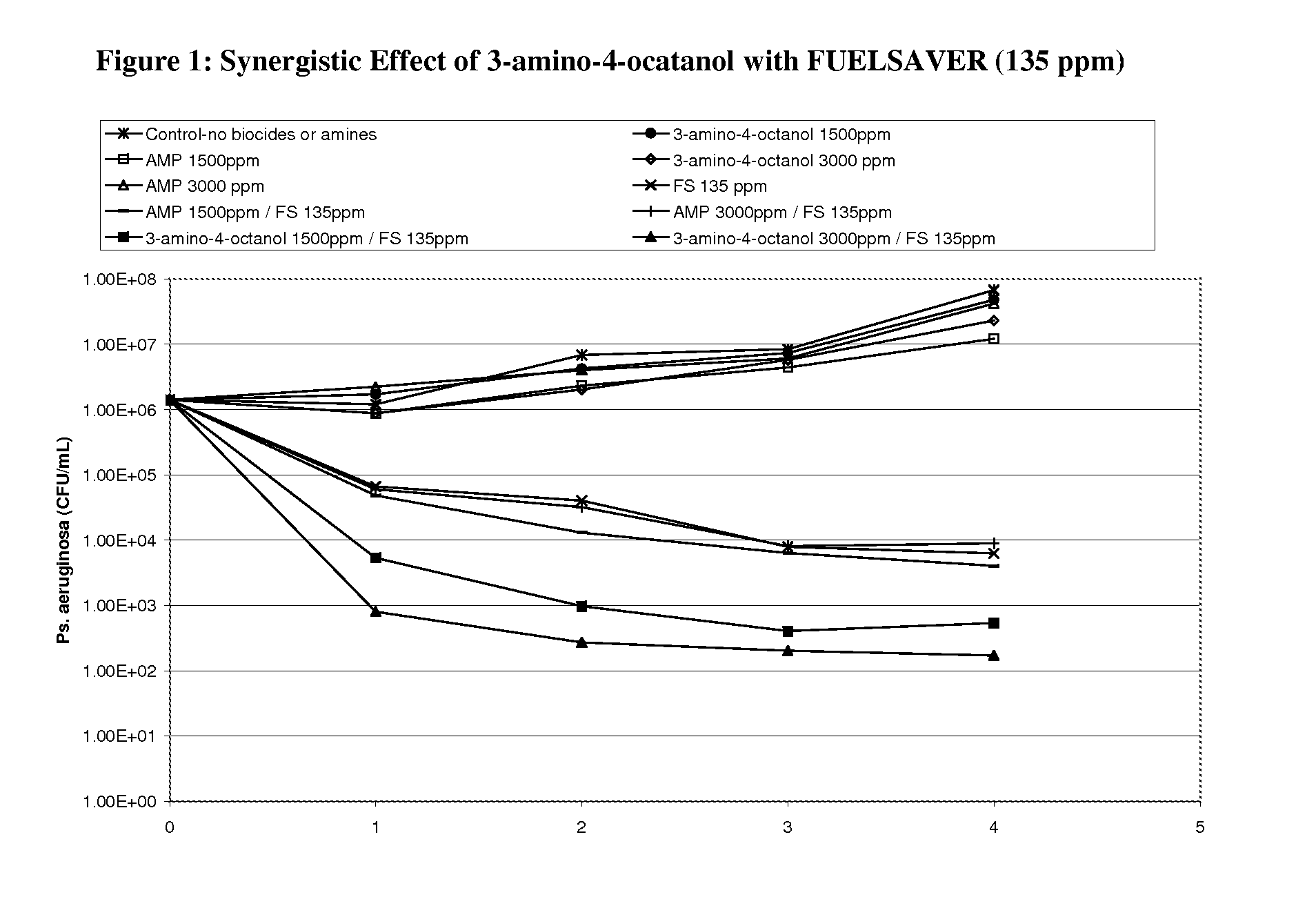
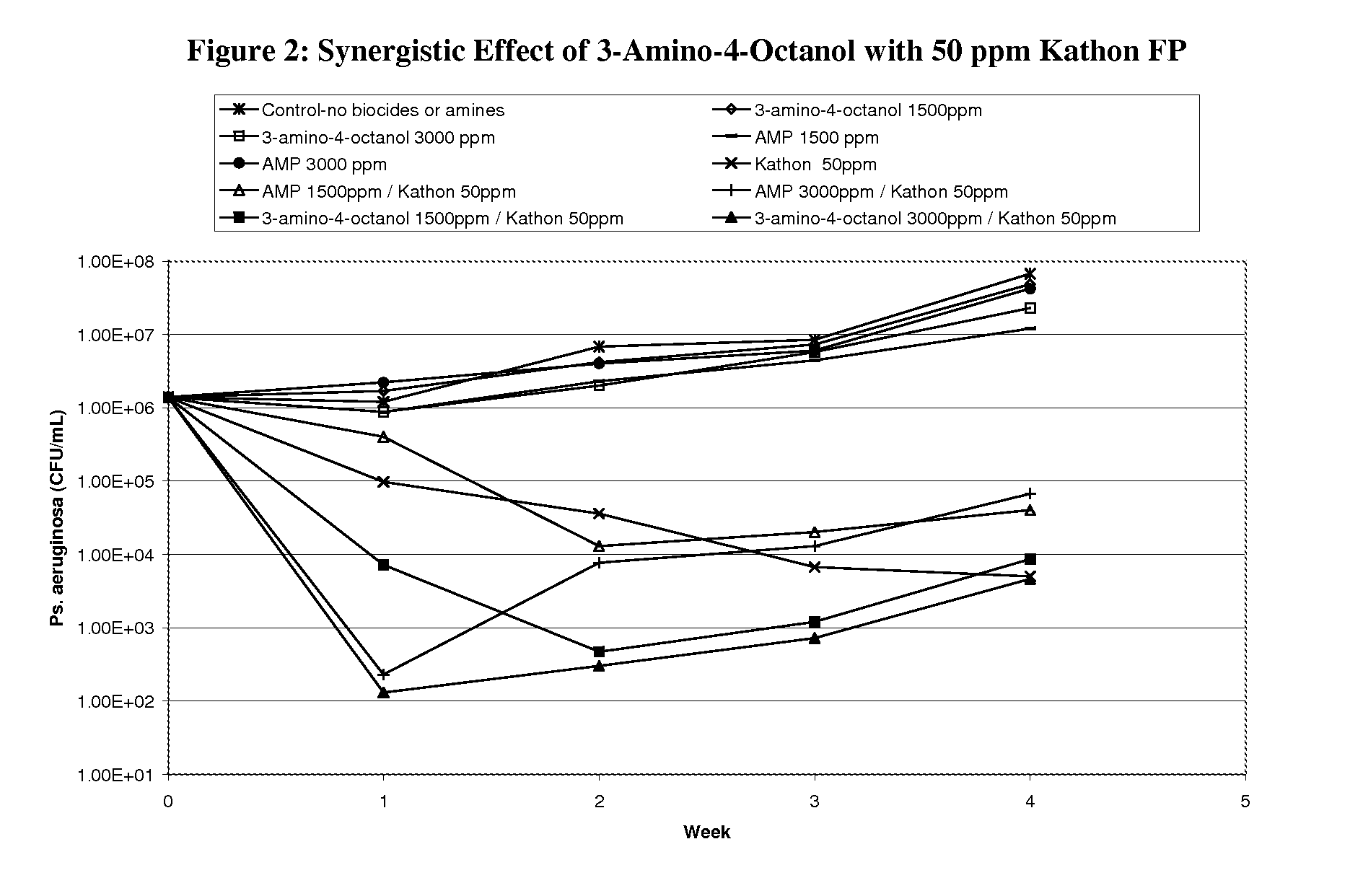
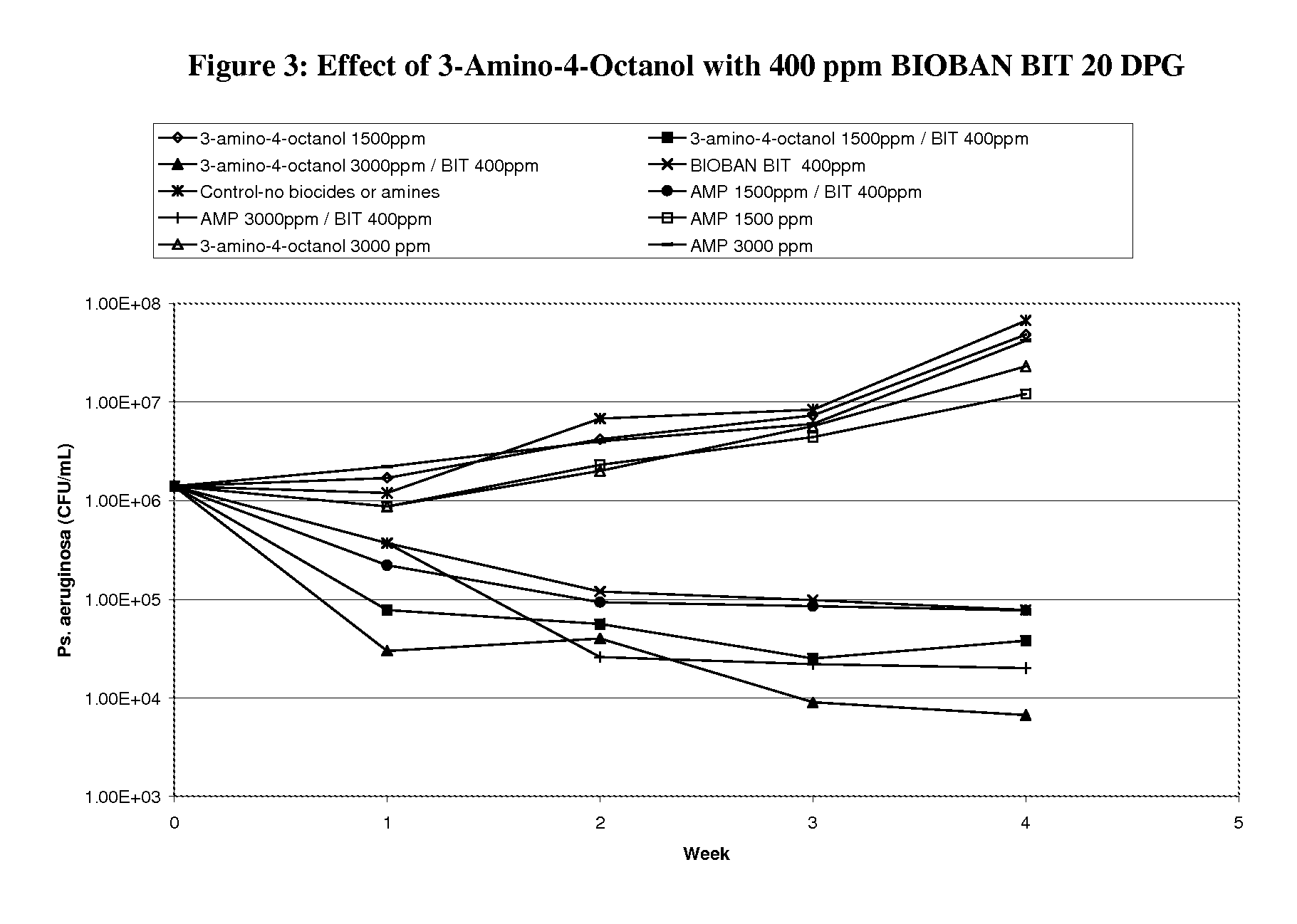
Corrosion and microbial control in hydrocarbonaceous compositions
a technology of hydrocarbonaceous compositions and corrosion control, applied in the direction of biocide, fuels, animal husbandry, etc., can solve the problems of fuel biodegradation, microbial contamination, and growth at the expense of hydrocarbon and non-hydrocarbon components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 3-amino-4-octanol
[0091]Preparation of the 3-nitro-4-octanol from 1-nitropropane and valeraldehyde. A sample of 3-nitro-4-octanol is synthesized by the addition of 1-nitropropane (1-NP, 300 g, 3.37 mols) into a 1 liter 3-necked round-bottomed flask (RBF, 24 / 40, 29 / 42, 24 / 40) equipped with a thermocouple, a magnetic stirrer, a 500 ml addition funnel, a nitrogen inlet, and a glass stopper. This light yellow liquid is diluted by the addition of methanol (MeOH, 150 g) that results in an endotherm. The caustic catalyst is added (16 g of a 10% aqueous solution and 0.60 g of a 50% aqueous caustic solution, 1.9 g total, 1.4 mole %). This changes the reaction color to orange and results in a slight exotherm. The valeraldehyde (258 g, 3.00 mols, 0.89 equivalents) is charged to the addition funnel and slowly added to the 1-NP over 3 h. The heat of reaction raises the temperature to 40-45° C. Once the valeraldehyde addition is complete, the contents of the RBF are transferred into...
example 2
Preparation 2-amino-3-heptanol
[0093]Preparation of the 2-nitro-3-heptanol from nitroethane (NE) and valeraldehyde. In a similar fashion as Example 1, a sample of 2-nitro-3-heptanol is synthesized by the addition of nitroethane (NE, 275 g, 3.67 mols) into a 1 liter 3-necked round-bottomed flask (RBF, 24 / 40, 29 / 42, 24 / 40) equipped with a thermocouple, a magnetic stirrer, a 500 ml addition funnel, a nitrogen inlet, and a glass stopper. The clear, colorless liquid is diluted by the addition of 95% ethanol (EtOH, 160 g) that results in an endotherm. The caustic catalyst is added (10 g of a 10% aqueous solution, 0.68 mole %) changing the reaction color to yellow and resulting in a slight exotherm. The valeraldehyde (258 g, 3.00 mols, 0.89 equivalents) is charged to the addition funnel and slowly added to the NE over 4 h. The heat of reaction raises the temperature to 40-45° C. Once the valeraldehyde addition is complete, the contents of the RBF are transferred into a 1 liter glass bottle,...
example 3
Preparation of 2-amino-2-methyl-3-heptanol
[0095]Preparation of the 2-methyl-2-nitro-3-heptanol from 2-nitropropane (2-NP) and valeraldehyde. In a similar fashion as example 1, a sample of 2-methyl-2-nitro-3-heptanol is synthesized by the addition of 2-nitropropane (2-NP, 300 g, 3.37 mols) into a 1 liter 3-necked round-bottomed flask (RBF, 24 / 40, 29 / 42, 24 / 40) equipped with a thermocouple, a magnetic stirrer, a 500 ml addition funnel, a nitrogen inlet, and a glass stopper. The clear, colorless liquid is diluted by the addition of absolute ethanol (EtOH, 150 g) that results in an endotherm. The caustic catalyst is added (16 g of a 10% aqueous solution and 0.6 g of a 50% aqueous solution, 1.4 mole %) changing the reaction color to light yellow and resulting in a slight exotherm. The valeraldehyde (258 g, 3.00 mols, 0.89 equivalents) is charged to the addition funnel and slowly added to the 2-NP over 3 h. The heat of reaction raises the temperature to 40-45° C. Once the valeraldehyde ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com