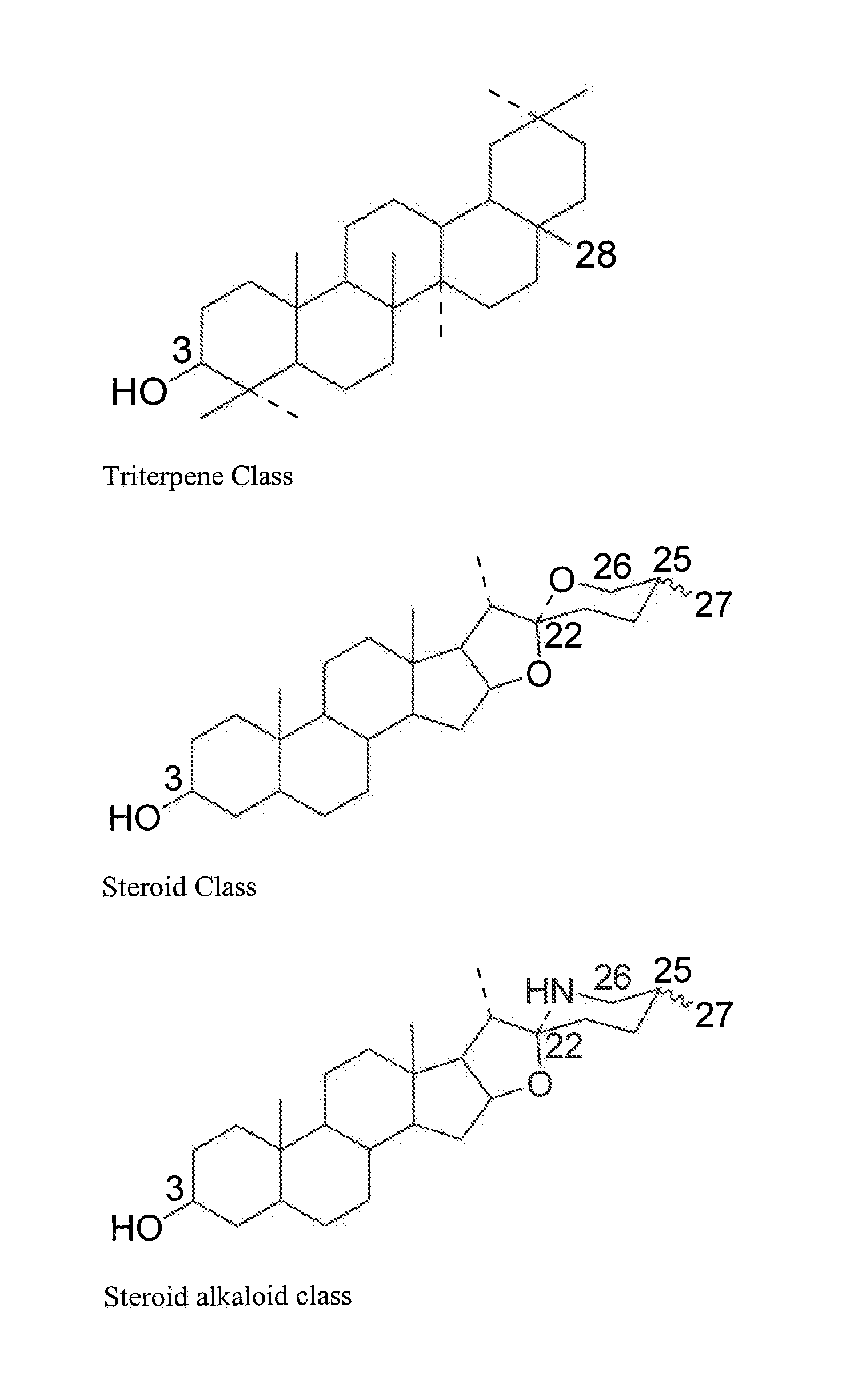
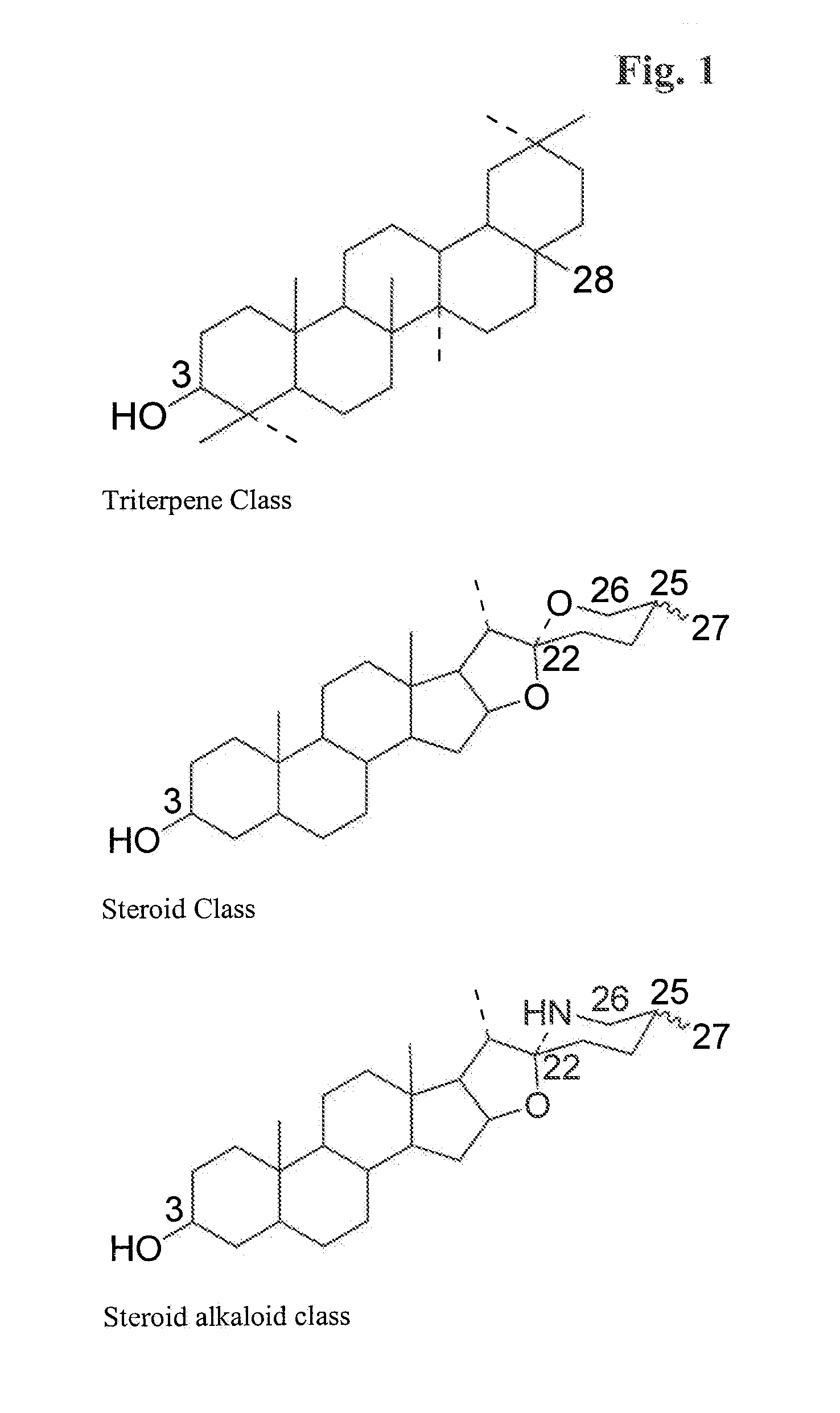
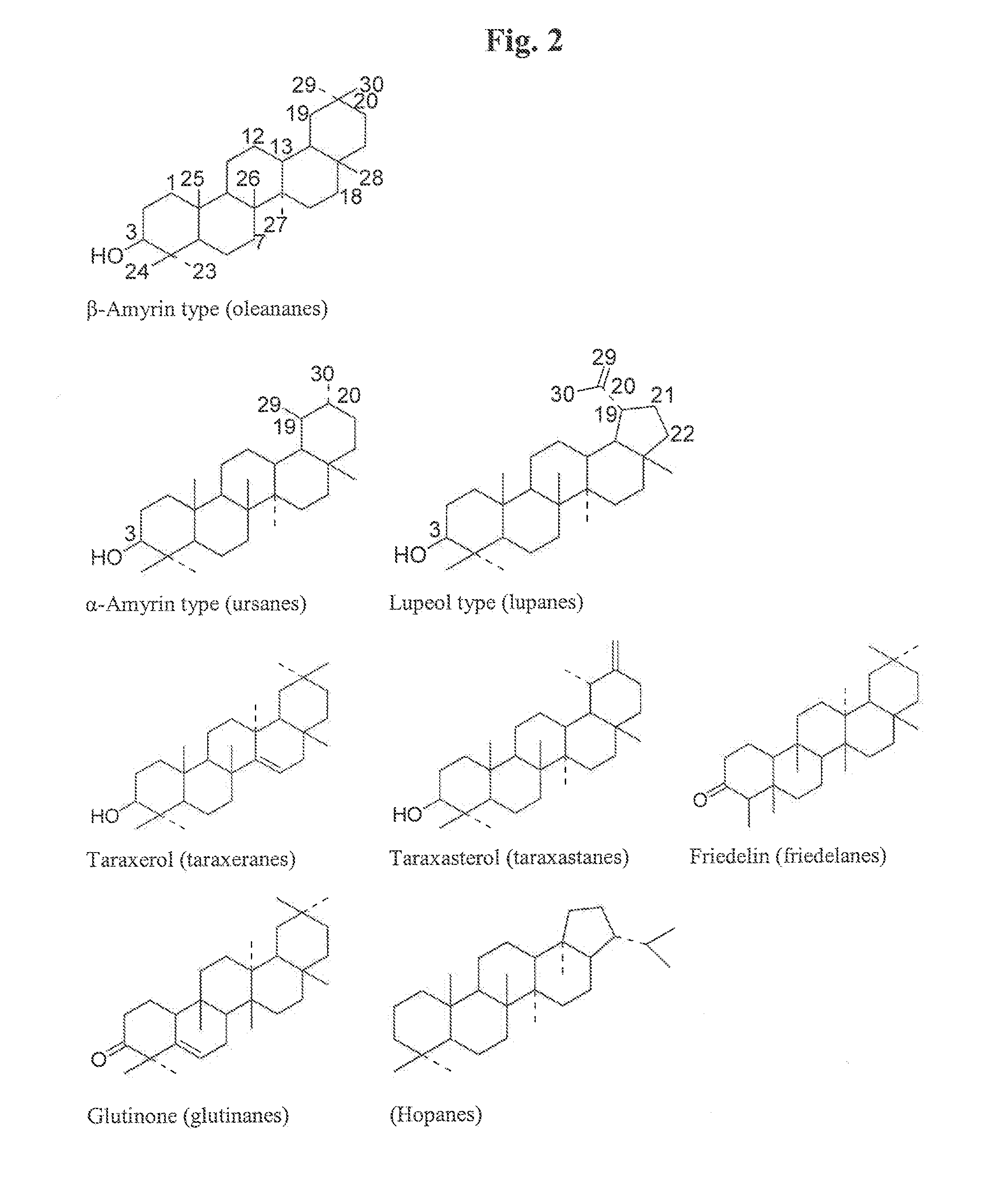
Particle structures comprising sterols and saponins
a technology of saponin and sterols, which is applied in the field of complexes of sterols and saponin glycosides, can solve the problems of not being able to remove by dialysis, unable to investigate the possibility that the detergent forms part of the iscom, and limited amount of intact iscom particles, so as to facilitate the uptake of polynucleotides and facilitate complex formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Incorporation of Low-Molecular Weight Substances into ISCOMs
[0489]Immune stimulating complexes (ISCOMs) are cage-like structures of uniform size with a diameter of approximate 40 nm. In general, the term ISCOM is used when antigen is inserted into the structures, whereas ISCOM-matrix denotes structures without antigen. Several reports describe the formation of ISCOMs with amphipathic proteins like virus membrane proteins. But also, non-amphipatic proteins like BSA and OVA as well as hydrophilic proteins linked to fatty acids have been used. Two crucial components for the formation of ISCOMs are cholesterol and phospholipid that are solubilized by detergent. Many protein antigens can be brought into solution by detergents compatible with the process of ISCOM-formation whereas other compounds like lipopeptides require different solvents. The methods reported here allow the embedment of such lipopeptides into the structures of ISCOMs by employing a pre-dissolution step in organic solve...
example 2
Preparation of DNA-Binding Complexes According to the Invention
[0539]This example demonstrates how to prepare micro-particles with the capability of forming an association with DNA by means of an electrostatic interaction The complexes were prepared by substituting a fraction of cholesterol for DC-cholesterol during the formation of the complexes.
[0540]Stock solution of sterol and phospholipid. Three lipid stock solutions was prepared by dissolving Cholesterol, 3β-[N-(N′,N′-Dimet-hylaminoethane)-Carbamoyl] Cholesterol (DC-Cholesterol, Avanti Polar Lipids) and phophatidylcholine (Epikuron 200S, Lucas Meyer Gmbh, Germany) in 20% w / v Mega 10 (N-Decanoyl-N-methyl-glucamide, Sigma-Aldrich). The concentration of the three stocks were (w / v):
DC-CholesterolCholesterolPhosphaditylcholineStock A0.5%0.5%1.0%Stock B0.25%0.75%1.0%Stock C0.13%0.87%1.0%
[0541]Lipids were dissolved by agitation and heating to 40° C. Stock solutions were stored at −20° C. until used.
[0542]Complex formation. A reaction...
example 3
Degradation of Linear Plasmid DNA Bound to DNA-Binding ISCOMs
[0546]The aim of the present example is to examine whether DNA associated with DNA-binding ISCOMs are protected from degradation by DNase I. Lineraized plasmid DNA was enzymatically labeled with 32P. Labeled DNA was allowed to bind to ISCOMs and treated with or without DNAse I. ISCOMs were collected by filtration and the amount of label retained was used as a measurement of the amount of DNA protected from degradation.
[0547]Labeling of DNA. Plasmid pUC18 digested with BamH1 (Amersham-Pharmacia, pUC18 BamH1 / BAP) was labeled using DNA polymerase I Kienow Fragment by adding 10 μCi [α-32P]dGTP (Amersham-Pharmacia, AA0066) to 1 μg of DNA in 25 μl EcoPol Buffer (New England BioLabs, 10 mM Tris-HCl, 5 mM MgCl2, 5 mM DTT, pH 7.5). By further addition of 1 unit Kienow Fragment (New England BioLabs, M0212S) the reaction was initiated. After incubation for 15 minutes at room temperature the reaction was stopped by heating for 5 minut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com