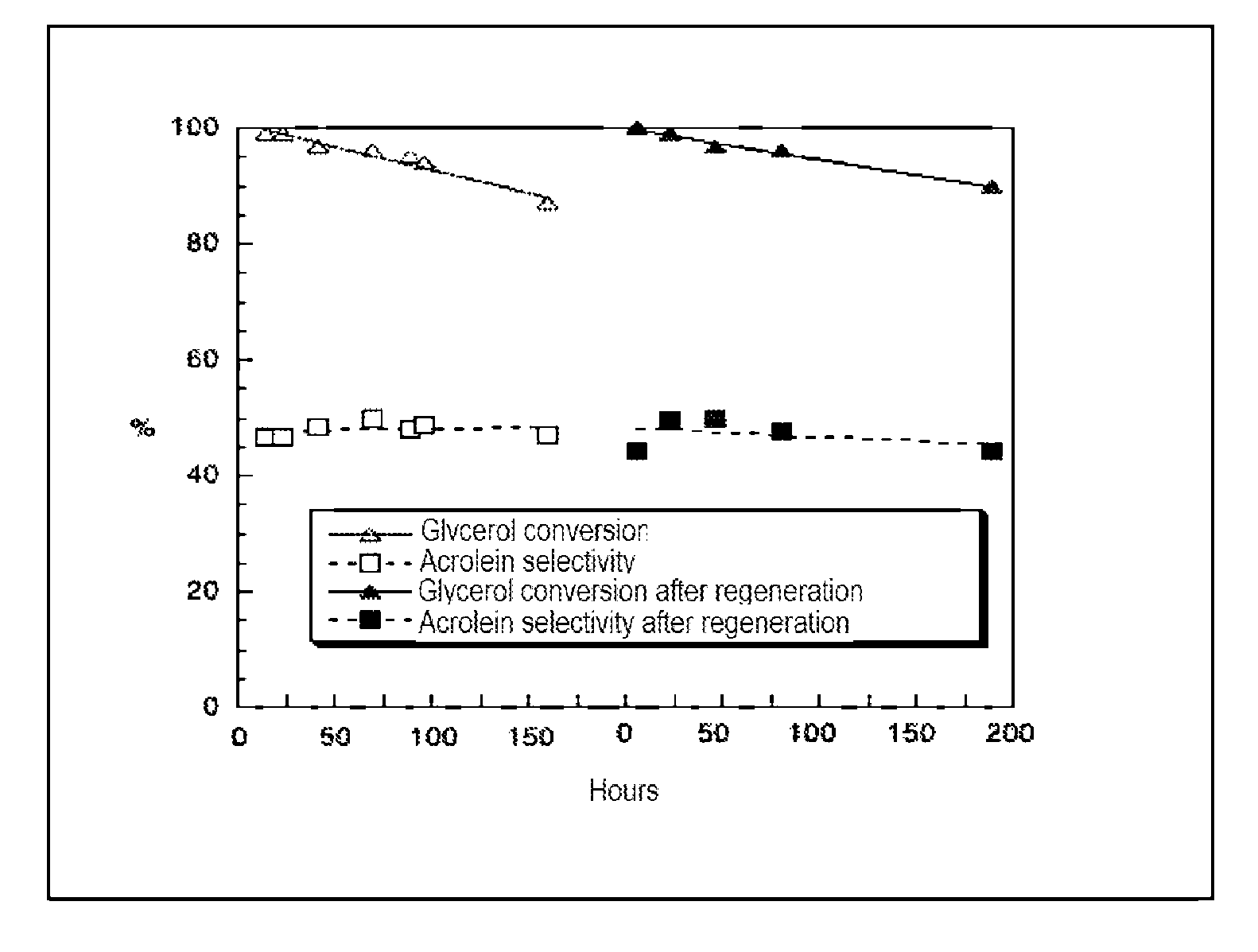
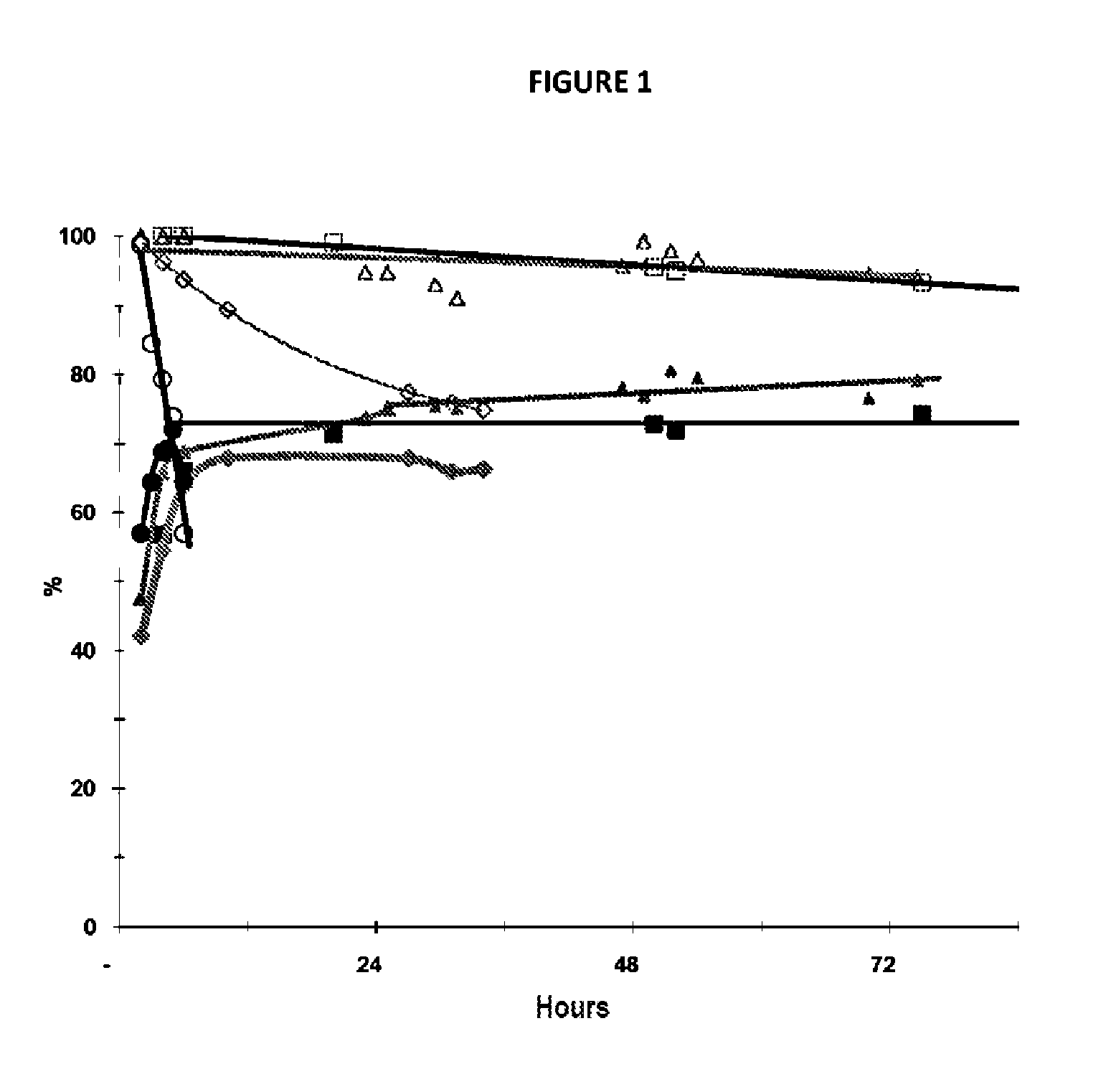
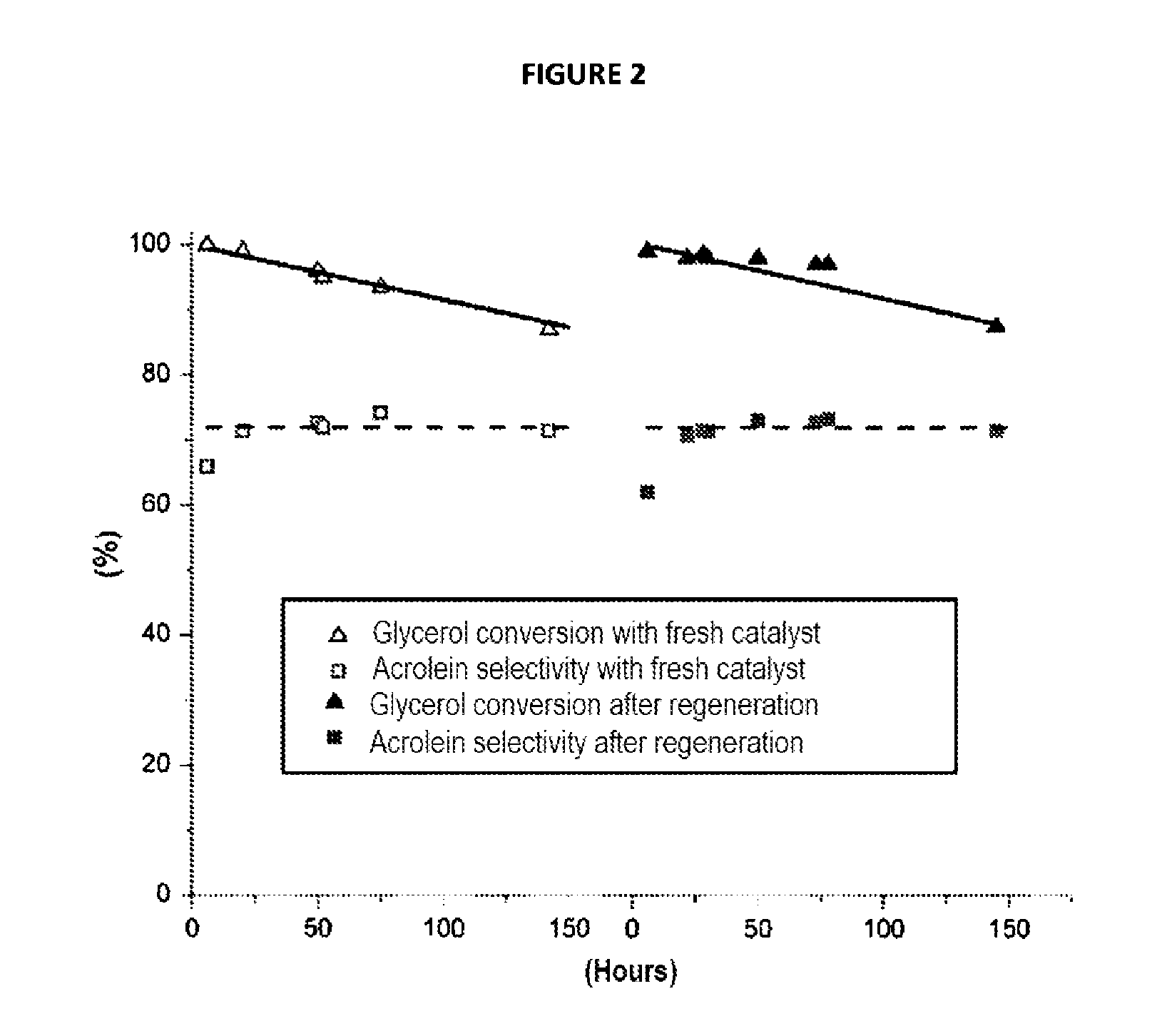
Method for preparing acrolein from glycerol or glycerine
a technology of glycerol or glycerine, which is applied in the direction of catalyst regeneration/reactivation, physical/chemical process catalysts, metal/metal-oxide/metal-hydroxide catalysts, etc., can solve the problems of not being able to absorb such a surplus, applications that are clearly insufficient for absorbing, etc., to avoid parasitic reactions and easy to adjust the concentration of glycerol in the solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation and Characterization of the Catalyst A
[0076]A catalyst according to the invention of the zirconium and niobium oxide type prepared from zirconium oxide hydrate and ammonium oxalate-niobiate, (NH4)(C2O4)2NbO.xH2O (Aldrich, 99.99%). The zirconium oxide hydrate is prepared by co-precipitation of a solution of zirconium oxonitrate ZrO(NO3)2.xH2O (Aldrich, 99%) and a 28% ammonia solution at pH=8.8.
[0077]The ammonium oxalate-niobiate is dissolved in permuted water acidified with concentrated HNO3 at pH˜0.5 and heated to 45° C. After returning to room temperature, the zirconium hydroxide hydrate is added in a ZrO2 / Nb2O5 molar ratio of 3:1, the hydration degree of the zirconium oxide hydrate is determined beforehand by thermogravimetric analysis (TGA). After 24 h with stirring, the mixture is filtered and the solid is calcined under air flow at 600° C. The specific surface area of this catalyst is 40 m2 / g. The specific surface areas of the solids were measured with the BET (Brun...
example 2
Preparation and Characterization of the Catalyst A′
[0078]A catalyst according to the invention of the zirconium and niobium oxide type is prepared according to the procedure described by Kantcheva. et. Al, Catalysis Communications (2009), 9(5), p 874-879, by impregnation of zirconium oxide hydrate.
[0079]The zirconium oxide hydrate was prepared by co-precipitation of a solution of zirconium oxonitrate ZrO(NO3)2.xH2O (Aldrich, 99%) and of a 28% ammonia solution. The precursor of Nb(V), (NH4)(C2O4)2NbO.xH2O (Aldrich, 99.99%) is added with stirring to a 35% hydrogen peroxide solution (Sigma Aldrich) acidified to pH≈0.5 with concentrated HNO3 and heated to 50° C. The H2O2 / oxalate molar ratio is 13 / 1. The solution is heated for 1 h at 50° C. before being cooled down to room temperature. Next, the zirconium oxide hydrate is again added while ensuring a ZrO2:Nb2O5 ratio of 6:1, the hydration degree of the zirconium oxide hydrate being determined by thermogravimetric analysis (TGA). The mixt...
example 3
Preparation and Characterization of the Catalyst E
[0081]A catalyst according to the invention of the zirconium and niobium oxide type is prepared according to the procedure described by Kantcheva. et. Al, (Catalysis Communications 9(5), (2008) p 874-879), by impregnation of zirconium oxide hydrate with a solution containing a mixed ammonium and niobium oxalate.
[0082]The precursor of Nb(V), (NH4)(C2O4)2NbO.xH2O (Aldrich, 99.99%) is added with stirring to a 35% hydrogen peroxide solution (Sigma Aldrich) acidified to pH˜0.5 with concentrated HNO3 and heated to 50° C. The H2O2 / oxalate molar ratio is 13 / 1. The solution is heated for 1 h at 50° C. before being cooled down to room temperature. Next, the zirconium oxide hydrate prepared beforehand by co-precipitation of a solution of zirconium oxonitrate (ZrO(NO3)s.xH2O (Aldrich, 99%) and of a 28% ammonia solution, is added while ensuring a ZrO2:Nb2O5 ratio of 6:1. The mixture is maintained with stirring at room temperature for 24 hrs and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com