Novel application of fibrinogen-420 and its active domain
a technology of fibrinogen and active domain, which is applied in the field of new fibrinogen420 and its active domain, can solve the problems of immune rejection reactions, monoclonal antibody technology, and ineffective preventive treatment or therapeutics for these diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
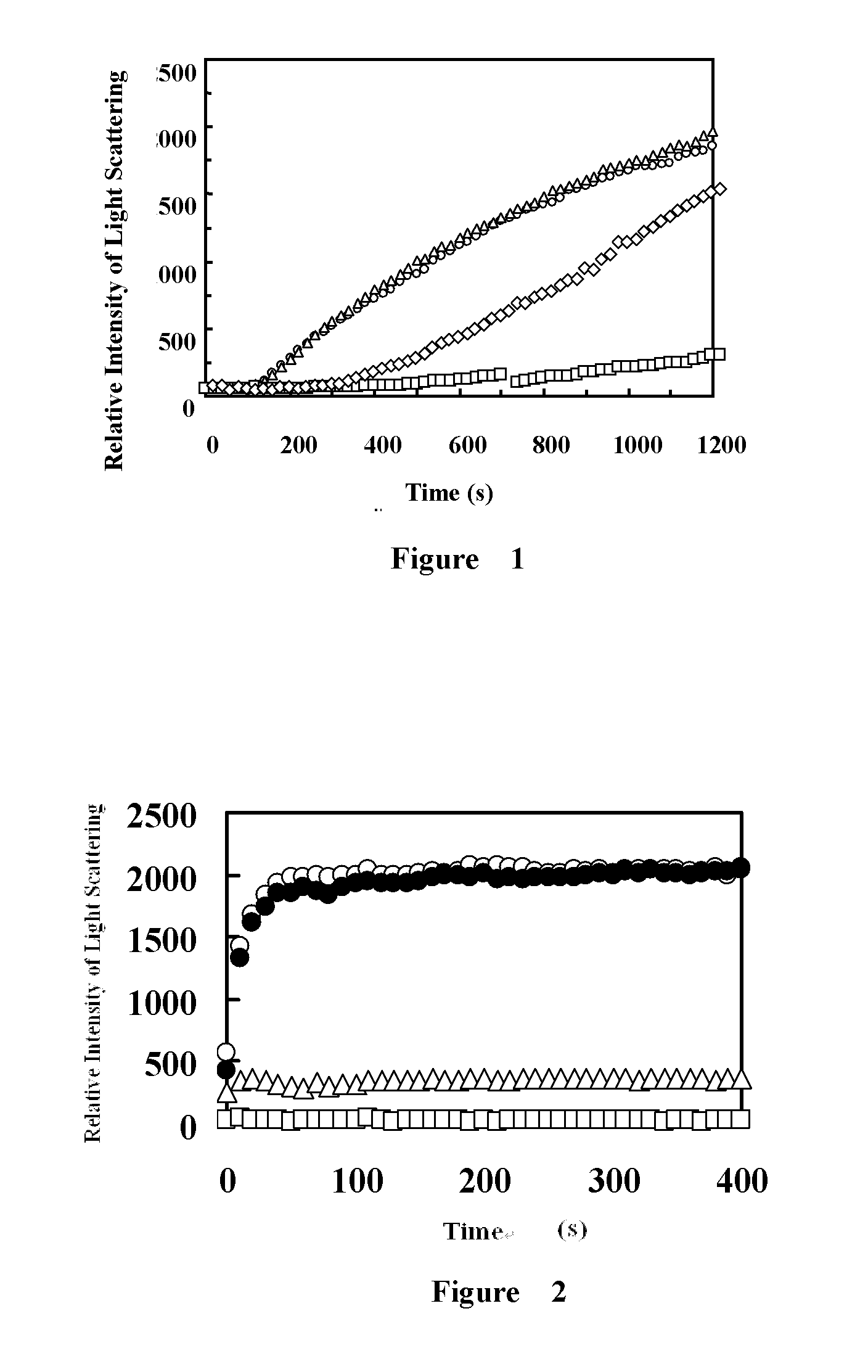
Fibrinogen-420 and Alpha EC Domain Protein Suppress Denatured Aggregation of Citrate Synthase
[0022]a. Preparation of Fibrinogen-420 and Alpha EC Domain Protein
[0023]The preparation of fibrinogen-420 started from the purification of a fibrinogen mixture from blood or cord blood, after which fibrinogen-420 can be further purified. Details are as follows:
(1) Purification of fibrinogen mixture: first, add protease inhibitors into fresh blood or cord blood, then centrifuge at 4° C. 2000 rpm and get yellow plasma from the supernatant. Then add glycine dry powder to the plasma while stirring to make glycine completely dissolved, and the final concentration of glycine is 2.1 M. After centrifugation at 5000 rpm for 15 min, the white flocculent precipitate is obtained. Dissolve the precipitate with buffer (0.15 M NaCl, 0.01 M sodium phosphate, pH 6.4 solution) that is ⅓ of the original plasma volume, and repeat this step until the dissolved volume is 1 / 10 of the original plasma volume. Add an...
example 2
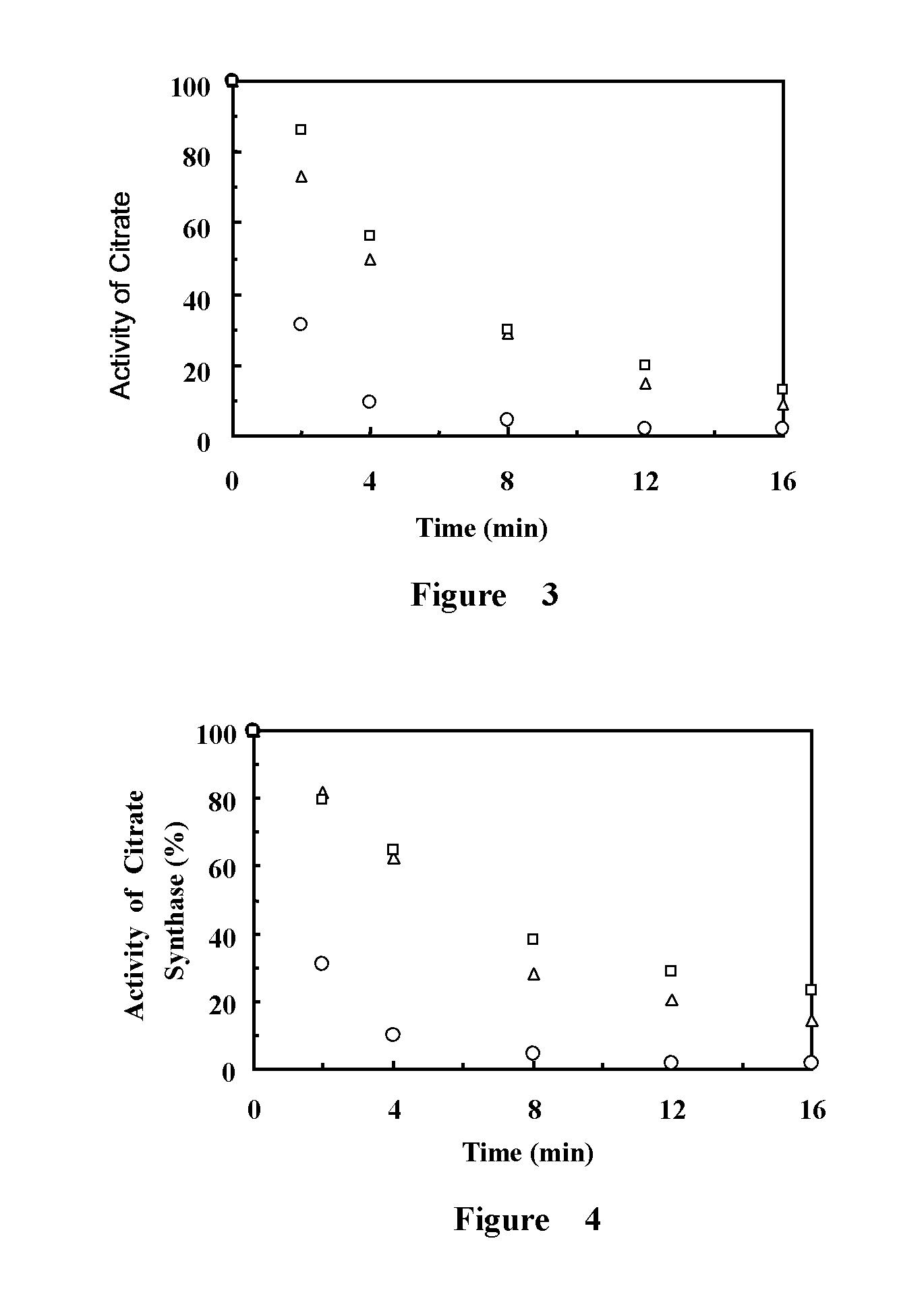
Fibrinogen-420 and Alpha EC Domain Protein Protecting the Activity of Citrate Synthase (CS)
[0031]The experimental method of fibrinogen-420 and alpha EC domain protein inhibiting CS inactivation of thermal denaturation is as follows:
[0032]Dissolving the citrate synthase in the 40 mM HEPES buffer solution and making the final concentration to 0.075 μM. Simultaneously, the experiment group 1 is added 0.075 μM fibrinogen-420, the experiment group 2 is added 0.15 μM fibrinogen-420, the experiment group 3 is added 0.15 μM, the experiment group 4 is added 0.15 μM alpha EC domain protein and the control group is added an equal volume of HEPES buffer solution. Putting the samples into the 43° C. water bath and beginning to detect the change of the activity of citrate synthase. The activity of citrate synthase is defined 100% before heating.
[0033]The method of detecting the activity of citrate synthase is as follows:
[0034]930 μL TE buffer solution (50 nM Tris, 2 mM EDTA, pH 8.0), 10 μL 10 mM ...
example 3
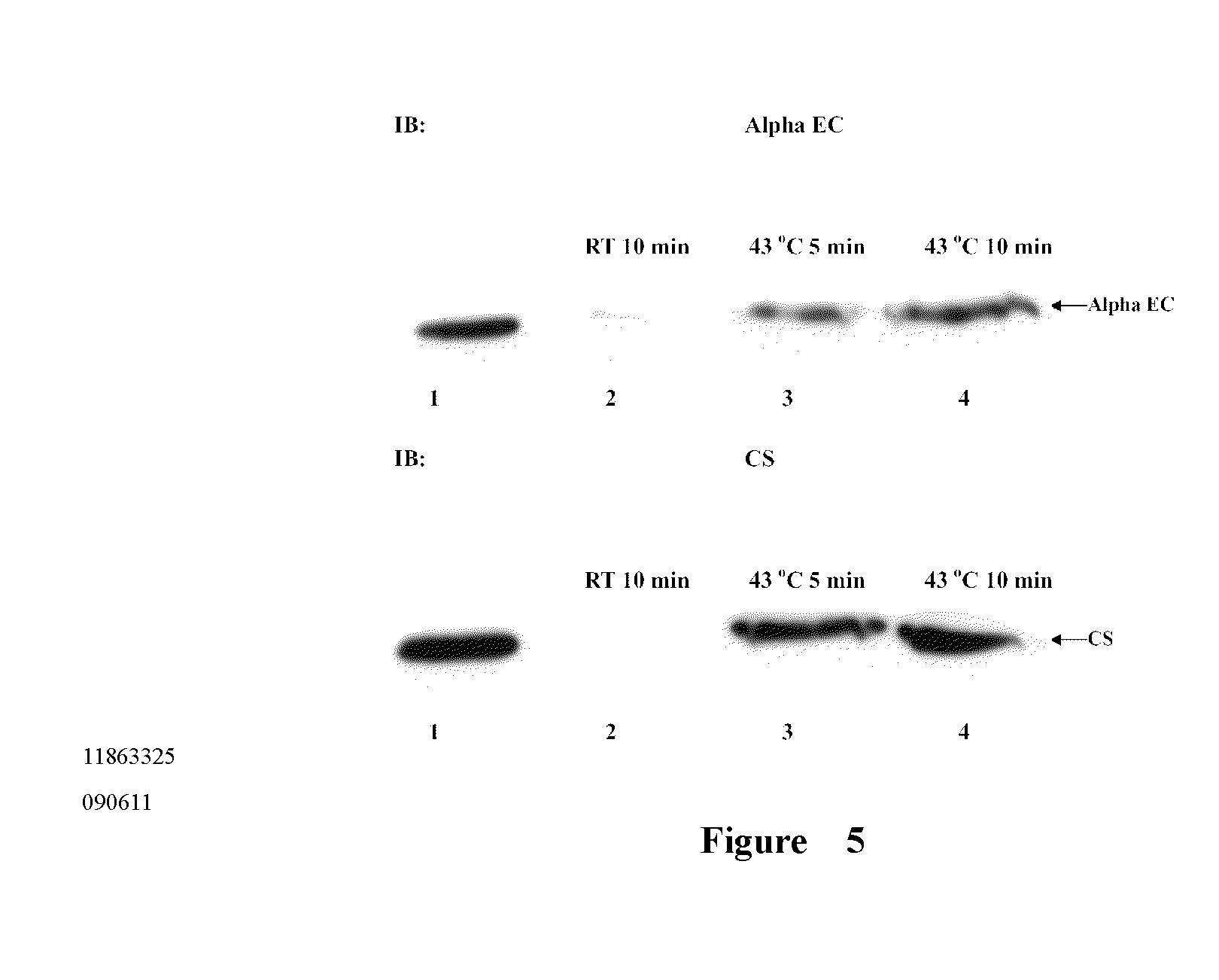
Alpha EC Domain Protein Recognizing Citrate Synthase Specifically
[0037]Citrate synthase and alpha EC domain protein are incubated together at 43° C. After being heated for 5 min or 10 min, antibodies of citrate synthase and alpha EC domain protein are added into the supernatant to perform co-immunoprecipitation. In the control group, citrate synthase and alpha EC domain protein are incubated together at room temperature and antibodies of citrate synthase and alpha EC domain protein are added into the supernatant to perform co-immunoprecipitation.
[0038]Results shown in the FIG. 5 indicate that after adding the antibody of citrate synthase, the denatured citrate synthase can be precipitated and alpha EC domain protein can also be precipitated at the same time. After adding the antibody of alpha EC domain protein, both of alpha EC domain protein and citrate synthase can be precipitated. The results above indicate that after being heated, citrate synthase and alpha EC domain protein can...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com