Methods for treating metabolic disorders using fgf
a metabolic disorder and metabolic technology, applied in the field of metabolic disorders treated using fgf, can solve the problems of high incidence of type 2 diabetes, high mortality, morbidity and healthcare expenditure, blood vessel and nerve damage, etc., and achieve the effect of high fa
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
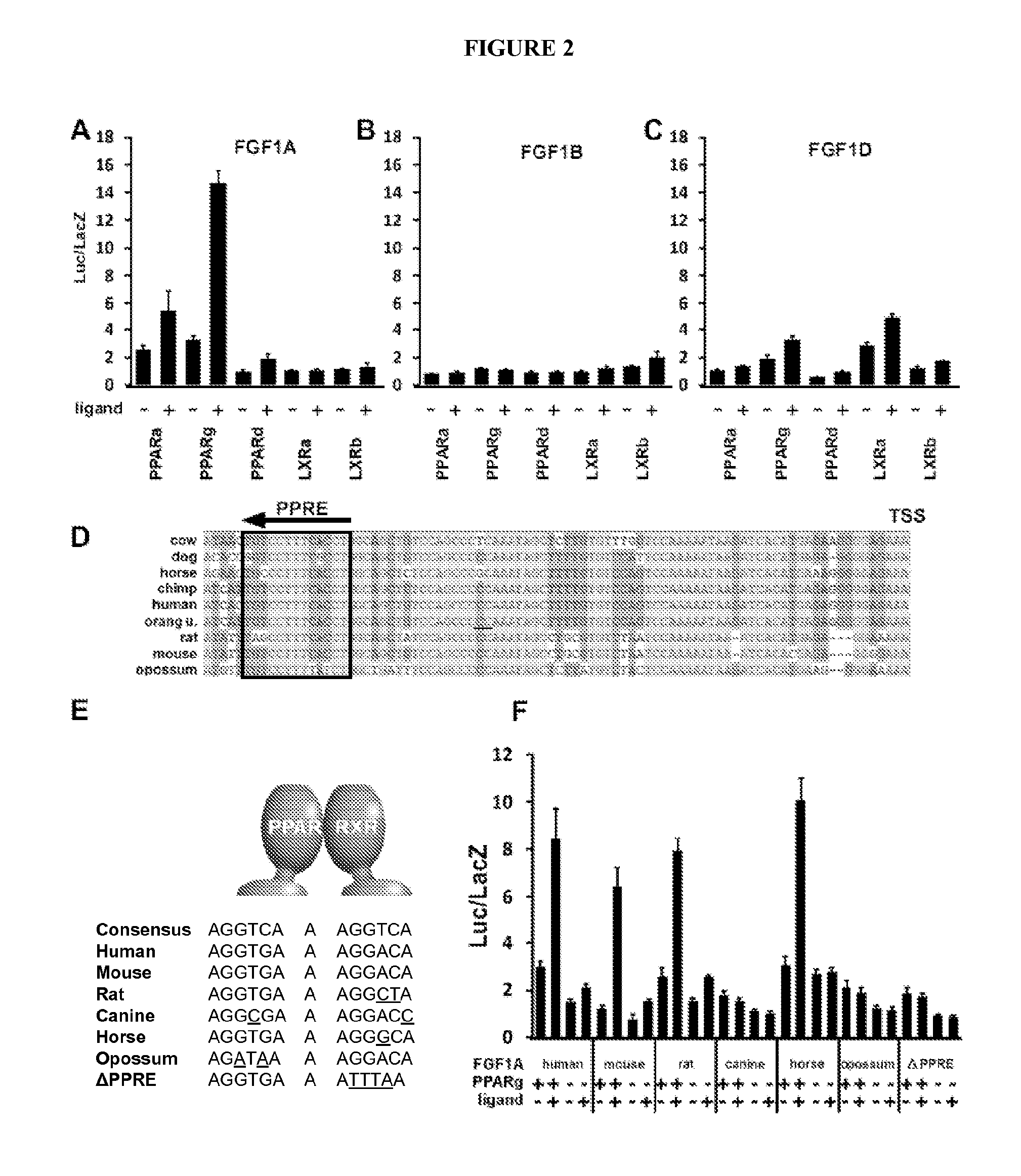
Identification of FGF-1 as a Direct Target of PPARγ
[0179]To identify nuclear hormone receptor (NHR) targets, we used a “Promoter Ontology” screen, which encompasses a validated cDNA expression library including all 49 mouse NHRs combinatorially paired with a large collection of pathway specific promoter-reporter libraries. The pairing facilitates rapid evaluation of the transcriptional regulation of each genetic pathway by any NHR in a given context. Using this high-throughput promoter screen, we screened promoter constructs for members of the FGF family for regulation by the NHRs, and identified FGF-1 as a direct target of PPARγ. More specifically, we identified strong and specific transcriptional regulation of FGF-1 by PPARγ.
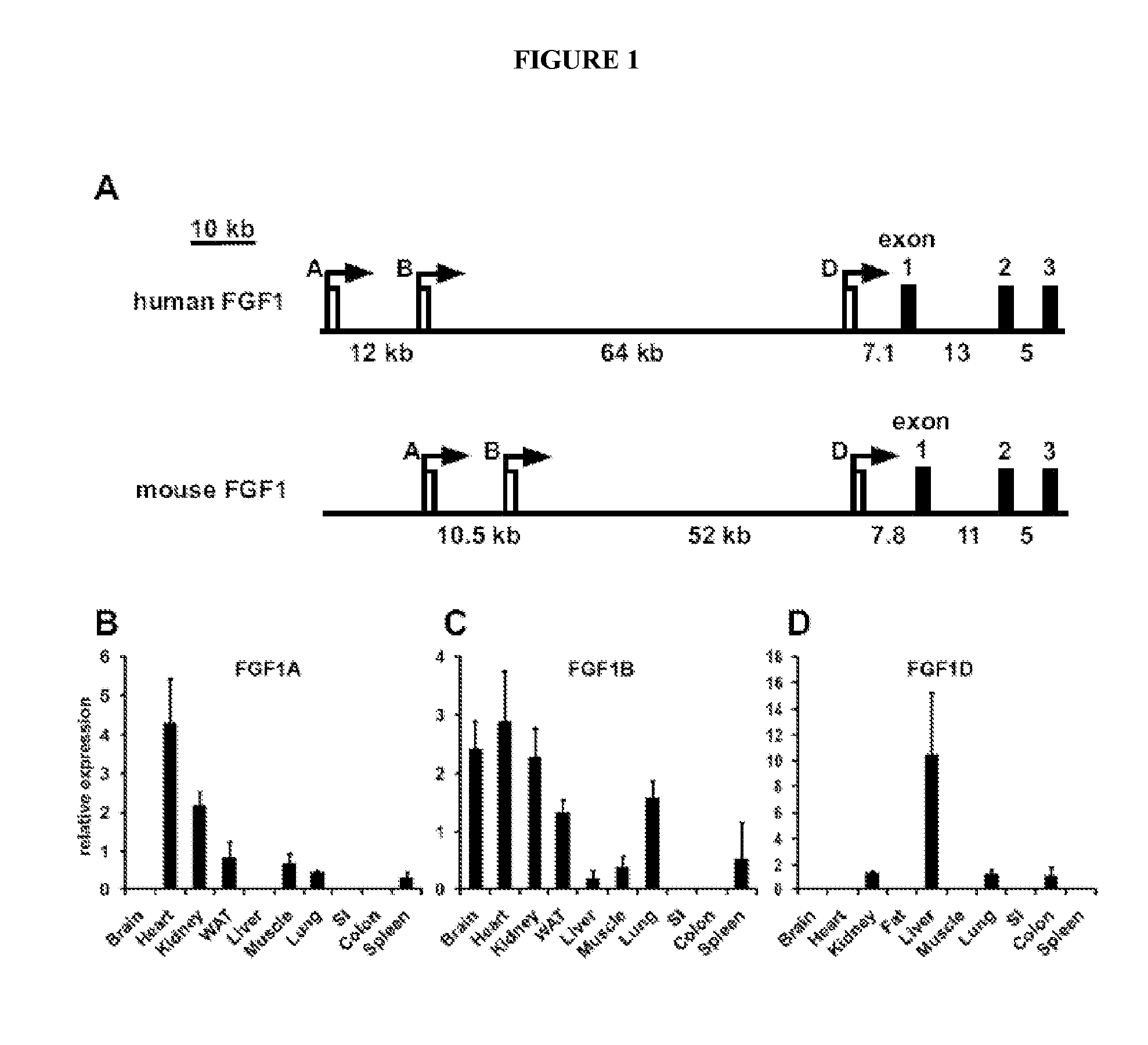
[0180]FGF-1A Promoter Characterization.
[0181]The expression of the FGF-1 gene is directed by at least three distinct promoters driving the untranslated exons: 1A, 1B, and 1D, spaced up to 70 kilobase pairs apart (FIG. 1A) (Myers et al., 1993). Alternative spli...
example 2
FGF-1 Protects Against HFD-Induced Insulin Resistance
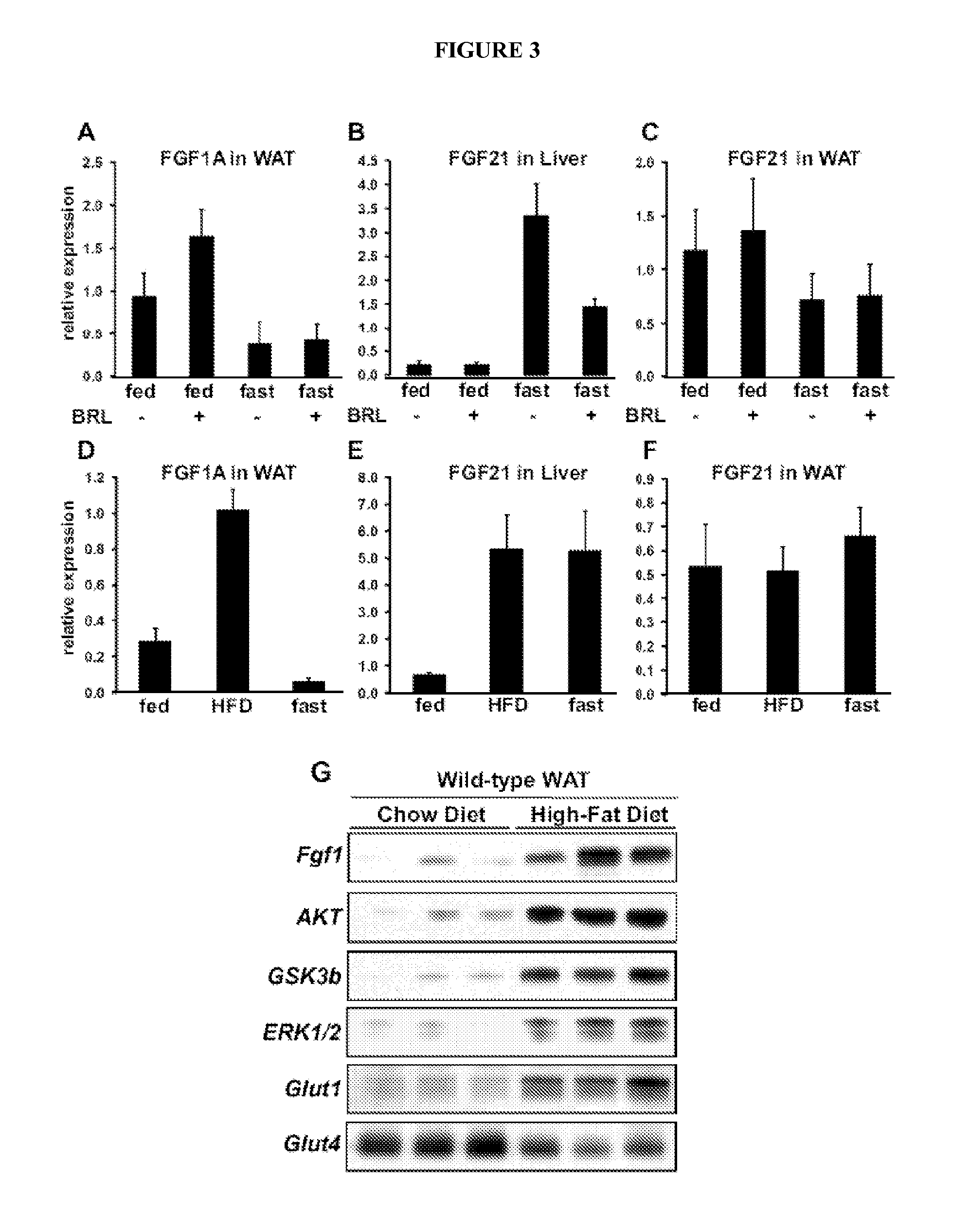
[0187]Next, we determined the consequences of loss of FGF-1 in vivo, using FGF-1 knockout (KO) mice. FGF-1 KO mice have been studied in the context of wound healing and cardiovascular changes. Neither these mice, nor FGF-1 / FGF2 double KO mice, displayed any significant phenotype under normal feeding conditions (Miller et al., 2000). To study the role of PPARγ-mediated regulation of FGF-1, FGF-1 KO and wild-type littermates were fed a high fat diet (HFD). Although no difference in HFD-induced weight gain was observed (FIG. 4A), FGF-1 KO mice had smaller WAT and larger, steatotic livers, suggesting that FGF-1 KO mice fail to increase their adipose mass and alternatively mobilize fat into the liver (FIG. 4B, C). At the same time, FGF-1 KO mice displayed increased fasting levels of glucose and insulin and increased insulin resistance compared to wild-type littermates as demonstrated by glucose- and insulin-tolerance tests (GTT, ITT), ...
example 3
AKT Signaling is Impaired in WAT of HFD-fed FGF-1 KO Mice
[0188]FGFs signal through four cognate high-affinity tyrosine kinase receptors, designated FGFR-1 to −4, leading to downstream activation of multiple signal transduction pathways, including the MAPK (ERK1 / 2) and PI3K / AKT pathways. These pathways regulate components of the insulin / glucose signaling pathways including activation of glycogen synthase kinase-3 (GSK-3), which regulates glycogen synthesis in response to insulin, and translocation of the glucose transporter GLUT4 (Cho et al., 2001). To investigate the integrity of these signaling pathways, we determined the expression of its critical components in WAT, BAT, liver, and muscle of HFD-fed FGF-1 KO and wild-type mice (FIG. 5). Interestingly, we found that total levels of AKT (and to a lesser extent GSK3(3) were reduced in WAT of HFD-treated FGF-1 KO mice compared to WT mice. In contrast, levels of AKT were normal in liver, BAT, or muscle, and levels of ERK1 / 2 were normal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com