Propionic acids, propionic acid esters, and related compounds
a technology of propionic acid and esters, applied in the field of new drugs, can solve the problems of uncontrolled drug use, faulty glutamate neurotransmission, and impaired cystine-glutamate antiporter activity, and achieve the effects of reducing the number of drugs, and improving the effect of drug resistan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthetic Strategies to Prepare Compounds of Formula (I)
[0079]
[0080]Pathway A: L-cysteine, a known compound, is reacted with a suitably substituted carboxylic acid, of formula (II), a known compound or compound prepared by known methods and a coupling agent such as EDC, HOBt, PyBROP, DCC, and the like, and a base, such as triethylamine, diisopropylethylamine, N-methylmorpholine and the like, in an organic solvent like tetrahydrofuran, N,N-dimethylformamide, dichloromethane, and the like, to give the compound of formula (III).
[0081]Pathway B: L-cysteine, a known compound, is reacted with a suitably substituted acyl halide, of formula (IV), a known compound or compound prepared by known methods and a base such as triethylamine, diisopropylethylamine, N-methylmorpholine and the like in an organic solvent like dichloromethane, tetrahydrofuran, toluene and the like, to give the compound of formula (III).
[0082]Pathway C: L-cysteine, a known compound, is reacted with a suitably substitute...
example 2
Synthesis of Ethyl (2R)-3-{[(2S)-2-Amino-3-Methylbutanoyl]Sulfanyl}-2-Acetamido-Propanoate Hydrochloride (AMRI-1)
[0086]
[0087]Ethyl (2R)-3-{[(25)-2-{[(tert-butoxy)carbonyl]amino}-3-methylbutanoyl]sulfanyl}-2-acetamidopropanoate. To a solution of L-N-Boc-valine (767 mg, 3.53 mmol), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (677 mg, 3.53 mmol) and hydroxybenzotriazole hydrate (477 mg, 3.53 mmol) in N,N-dimethylformamide (2.25 mL) was added 2-acetylamino-3-mercapto-propionic acid ethyl ester (450 mg, 2.35 mmol) and the resulting slurry was stirred at ambient temperature for 72 hours during which time a solution formed. The mixture was diluted with aqueous hydrochloric acid (0.2 M, 300 mL) and extracted with ethyl acetate (4×200 mL). The combined organic layers were washed with saturated aqueous sodium hydrogen carbonate (150 mL). The organic layer was dried over magnesium sulfate, filtered and concentrated at reduced pressure. This crude product was purified with sil...
example 3
In vitro Studies
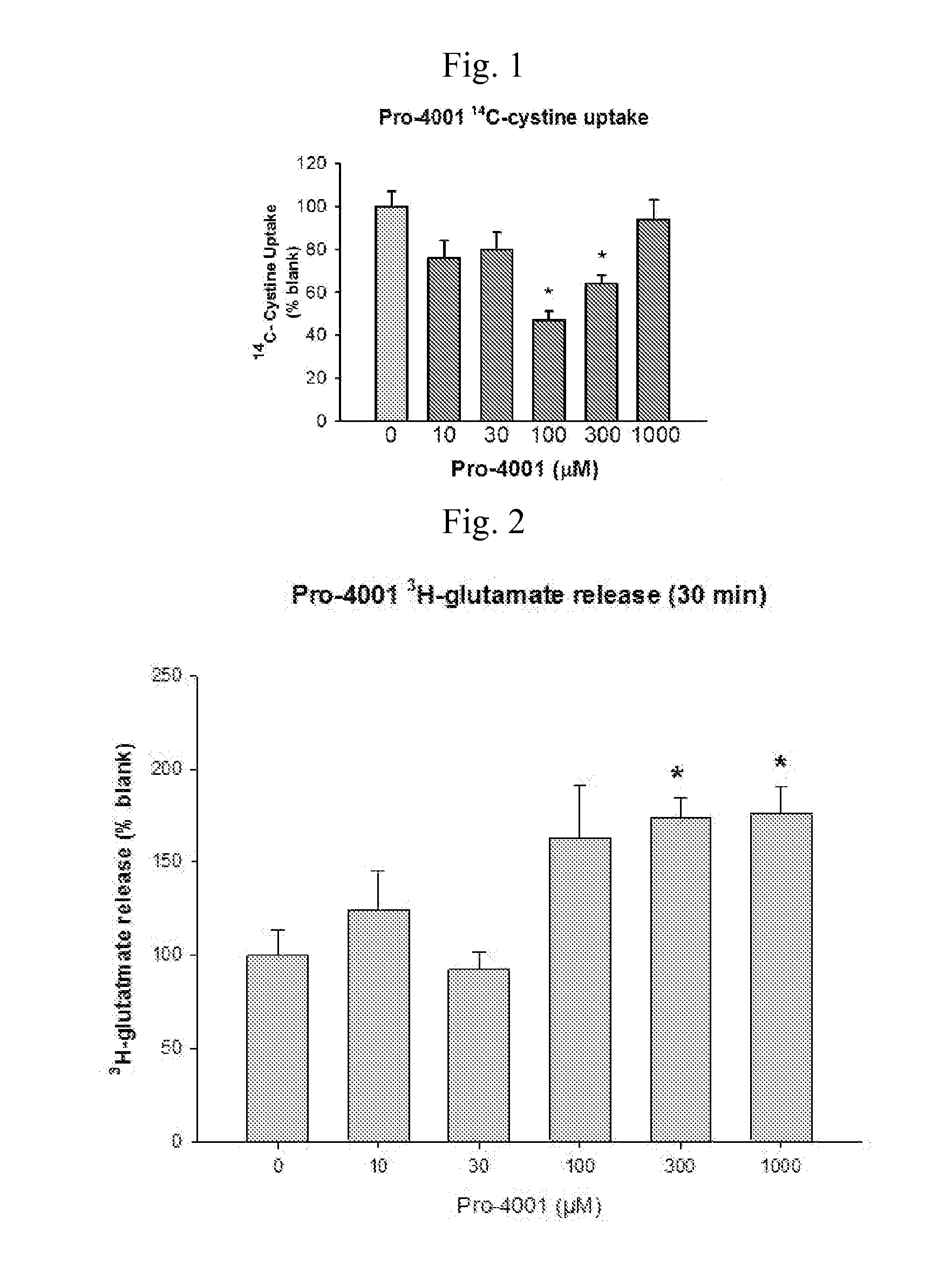
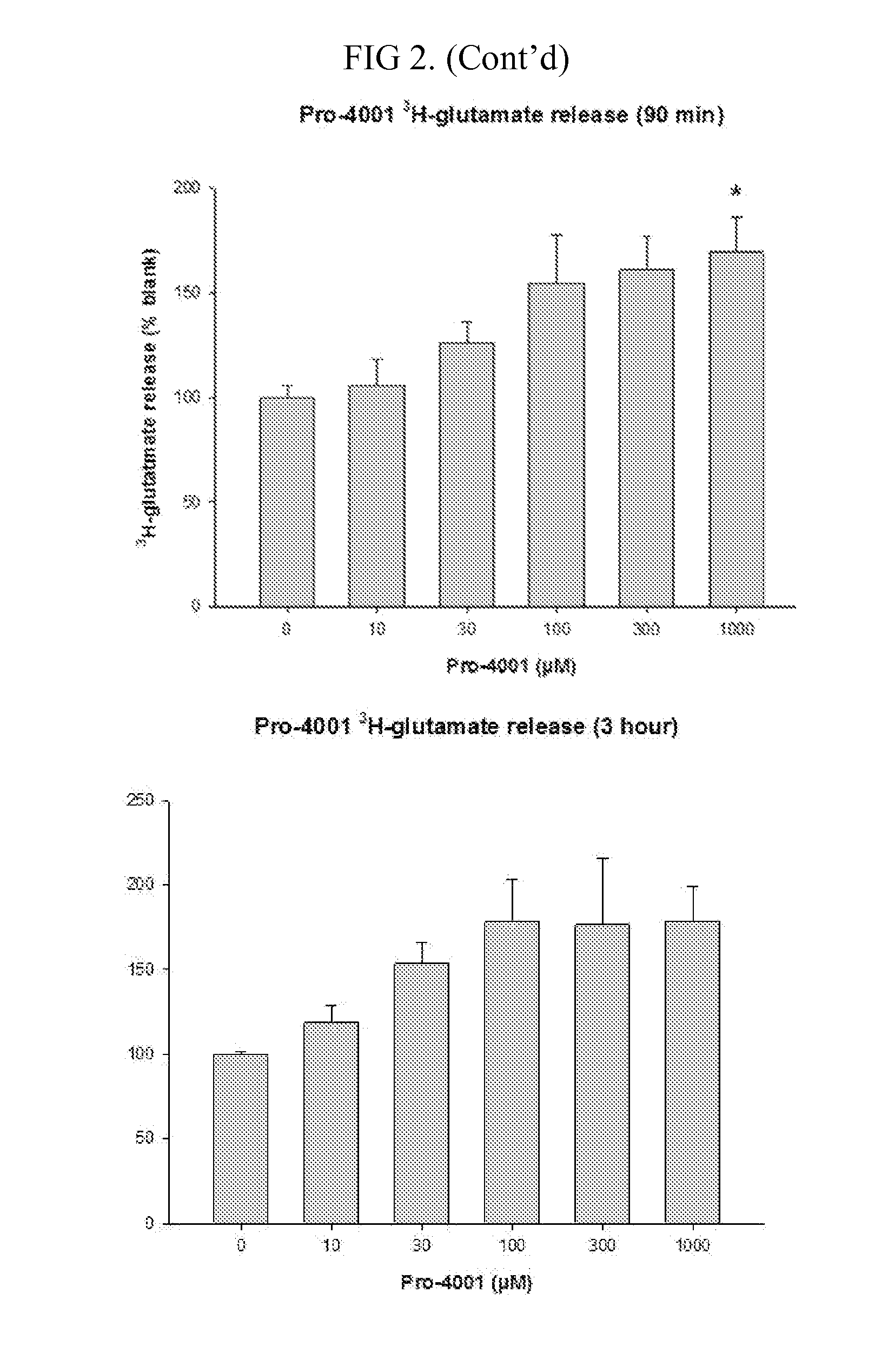
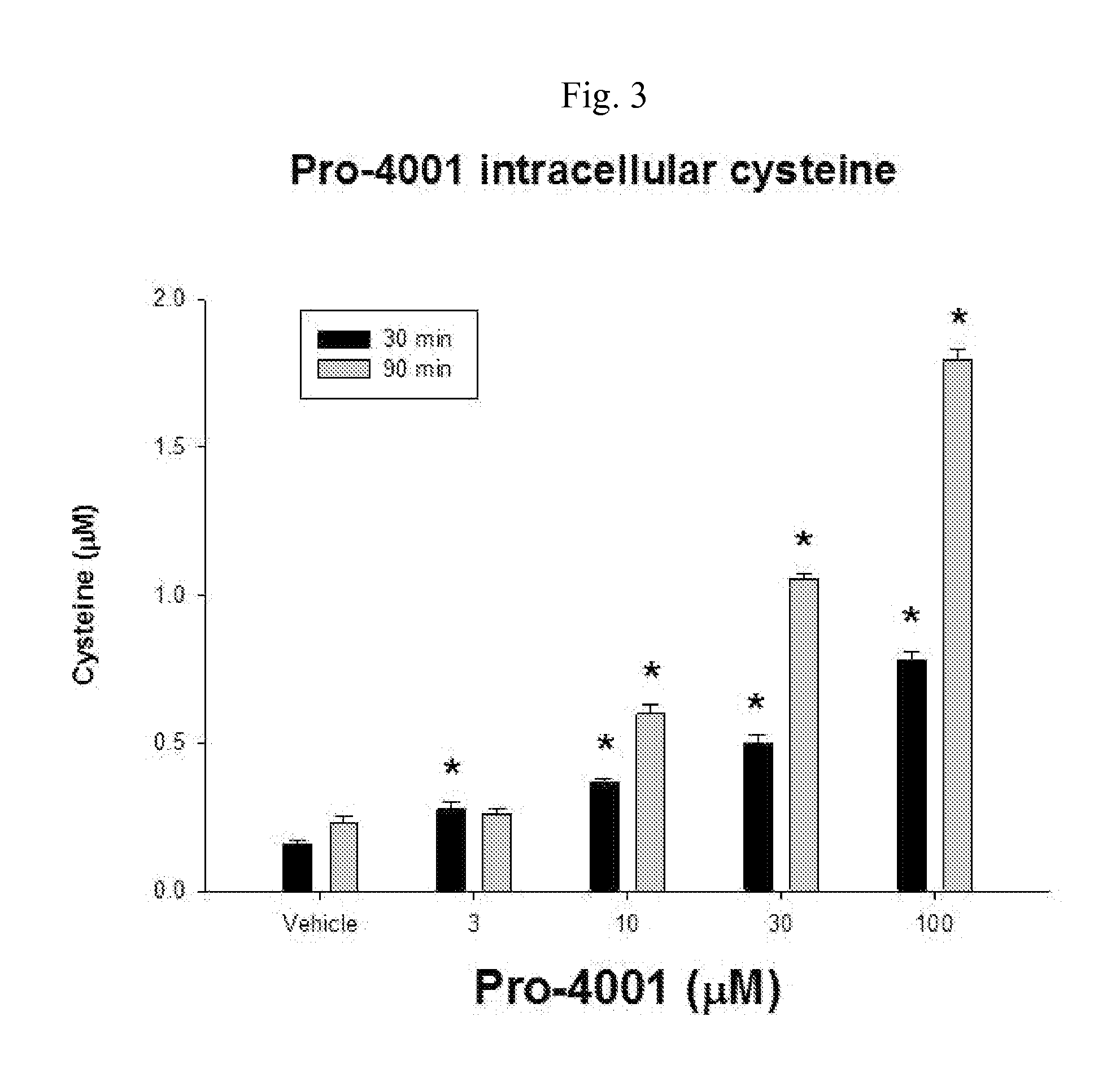
14C Uptake by Compound of the Invention
[0089]The goal of these experiments was to determine 14C-cystine uptake.
[0090]The experiments were conducted as follows.
[0091]The screening of the compound was performed using an in vitro culture system of human glial cells from brain astrocytoma (1321N1). Cells were plated on 24 well plates coated with poly-D-lysine and laminin and grown in a balanced salt solution supplemented with 5% heat inactivated horse serum, 5% fetal bovine serum, 2 mM glutamine and glucose (total 21mM). Cultures were maintained in humidified 5% CO2 incubators at 37° C. for 3-4 days before experiments were performed, at this time the cultures has formed a single confluent layer. For experiments, cultures are washed 3 times into a Na-free HEPES and HCO3− buffered balanced salt solution. After 1 hour, the test compounds are added. Following a three hour incubation, 14C-cystine (0.025 mCi / mL) was then added for 20 minutes. Following the 14C-cystine exposure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
| oxidative stress | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com