Cyanide-free electroplating baths for white bronze based on copper (i) ions
a technology of white bronze and cyanide, applied in the field of cyanide-free electroplating baths for white bronze based on copper (i) ions, can solve the problems of toxic white bronze, low current efficiency ranging from 50% to 80%, and relatively slow plating speed of 0.1, so as to achieve high current efficiency, high plating speed, good ductility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Ternary White Bronze of Copper / Tin / Silver
[0039]The following aqueous acid white bronze electroplating bath was prepared:
TABLE 1CONCENTRATIONCOMPOUND(g / L)Copper (I) ions as copper oxide30Tin (II) ions as tin methane sulfonate12Silver (I) ions as silver methane sulfonate51-(2-dimethylamino-ethyl)-5-mercapto-961,2,3,4-tetrazole3,6-dithia-1,8-octanediol75Methane sulfonic acid (70%)150g / LAntimony as potassium antimony tartrate0.16Nonionic phenol ethoxylate10.8Hydroquinone monosulfonic acid1g / L1Adeka Tol PC-8: non-ionic surfactant, available from Adeka Corporation.
[0040]The pH of the bath was less than 1 as measured using a KNICK Instruments conventional laboratory pH meter. The molar mass of the tetrazole compound, 3,6-dithia-1,8-octanediol and copper (I) ions was 173.24, 182.30 and 63.55 g / mol, respectively. The mole ratio of the tetrazole to the cooper (I) ions in the bath was 1.2:1 and the mole ratio of the tetrazole to the 3,6-dithia-1,8-octandiol was 1.3:1.
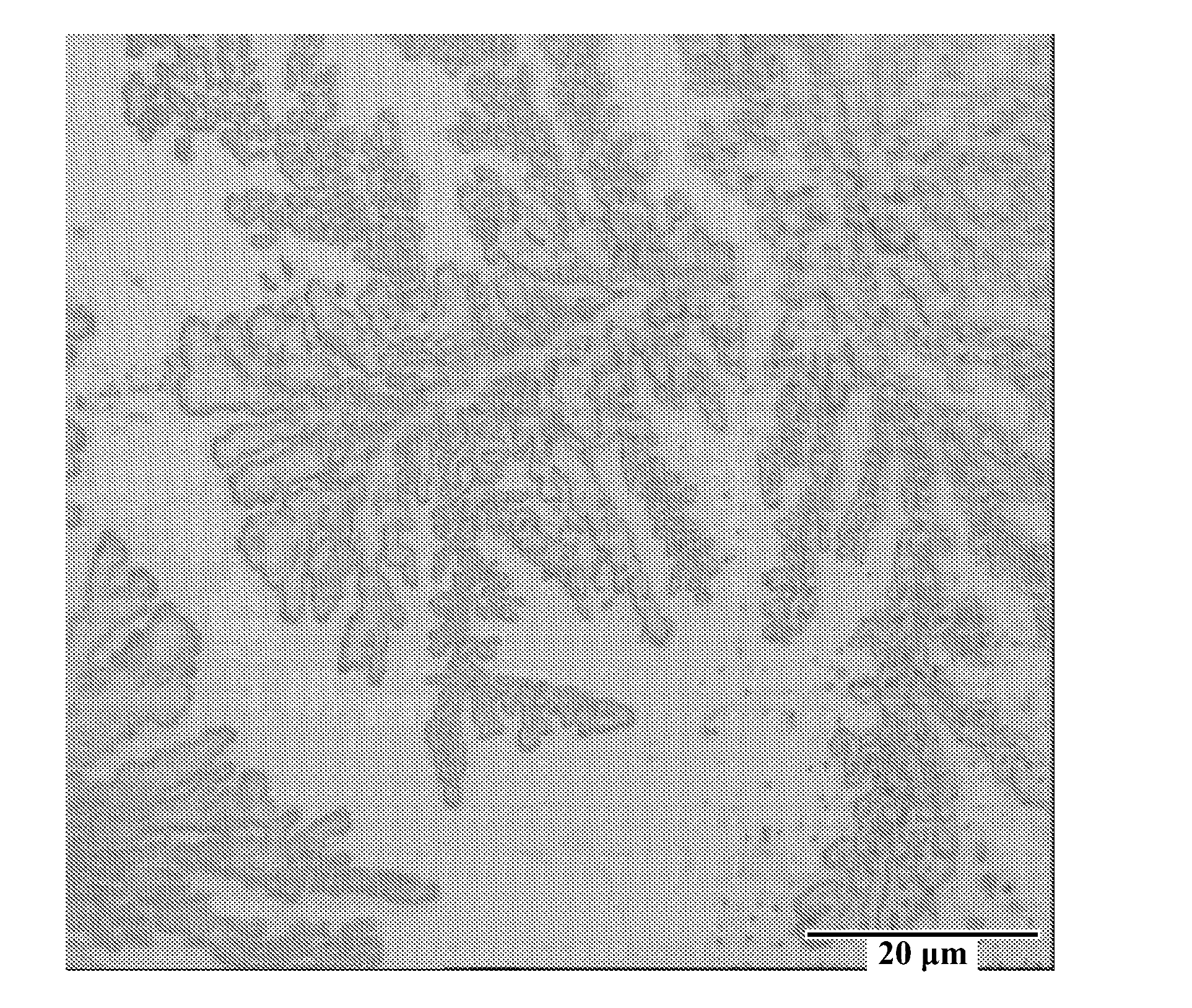
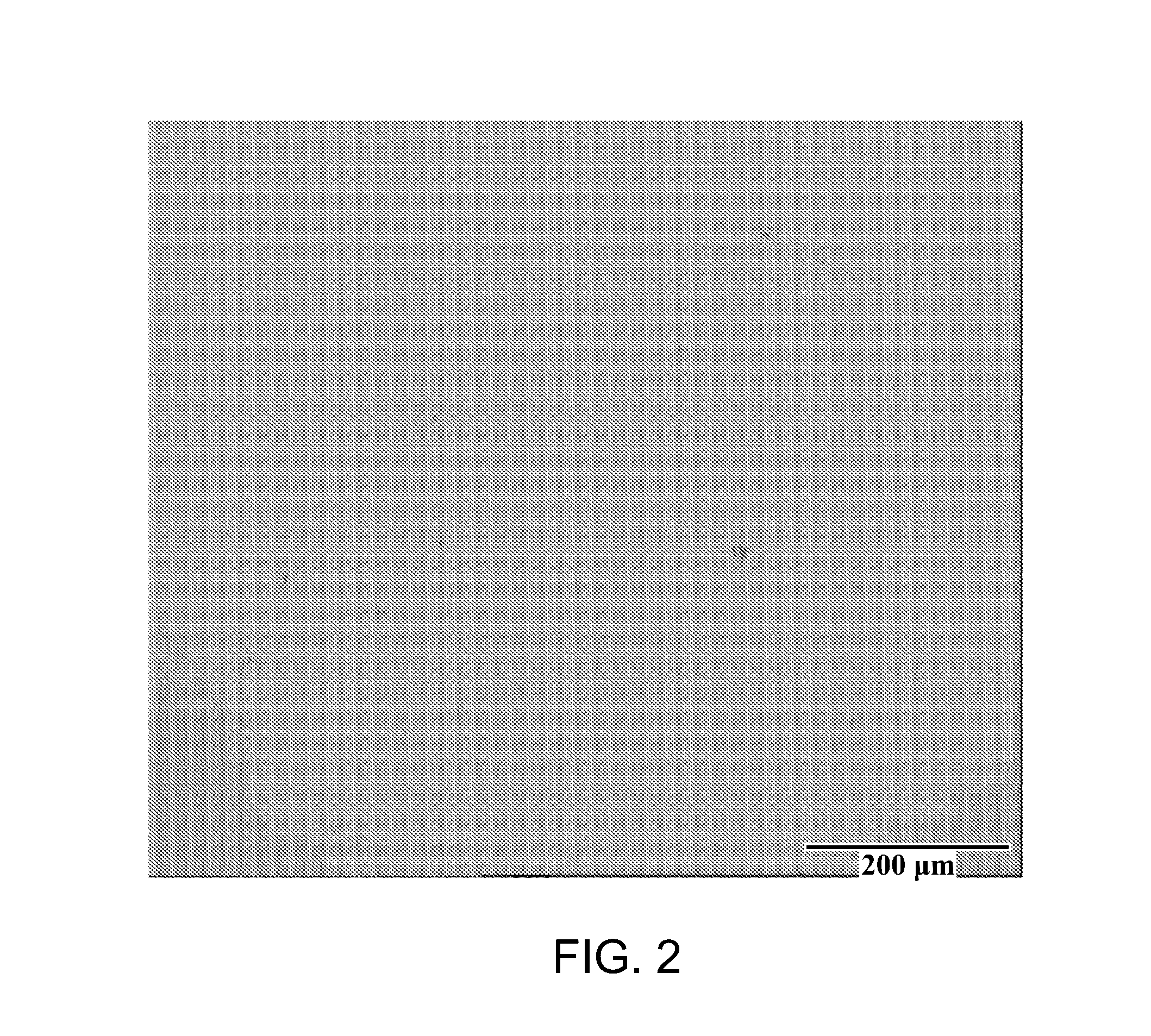
[0041]A brass panel having...
example 2
Binary White Bronze of Copper / Tin
[0043]The following aqueous acid white bronze electroplating bath was prepared:
TABLE 2CONCENTRATIONCOMPOUND(g / L)Copper (I) ions as copper oxide14Tin (II) ions as tin methane sulfonate81-(2-dimethylamino-ethyl)-5-mercapto-421,2,3,4-tetrazoleThiodiethanol80Hydroquinone monosulfonic acid1.6g / LMethane sulfonic acid (70%)90g / LBismuth methane sulfonate0.021,10-Phenanthroline monohydrate0.01Nonionic phenol ethoxylate20.82Adeka Tol PC-8: non-ionic surfactant, available from Adeka Corporation.
[0044]The pH of the bath was less than 1 as measured using a KNICK Instruments conventional laboratory pH meter. The mole ratio of the tetrazole to the cooper (I) ions in the bath was 1.1:1 and the mole ratio of the tetrazole to the thiodiethanol was 0.4:1.
[0045]A brass panel having dimensions 10×7.5×0.025 cm was degreased cathodically at 4 ASD for 1 minute using RONACLEAN™ DLF solution and activated by immersing the substrate for 20 seconds in RONASALT™ 369 solution. Th...
example 3
Tetrazole / 3,6-Dithia-1,8-Octanediol Mole Ratio in a Copper / Tin / silver Electroplating Bath
[0047]The white bronze copper / tin / silver alloy electroplating bath was prepared as described in Example 1 with the exception that the amount of 3,6-dithia-1,8-octanediol was varied as shown in Table 3 below. The mole ratio of 1-(2-dimethylamino-ethyl)-5-mercapto-1,2,3,4-tetrazole to 3,6-dithia-1,8-octanediol was as shown in Table 3.
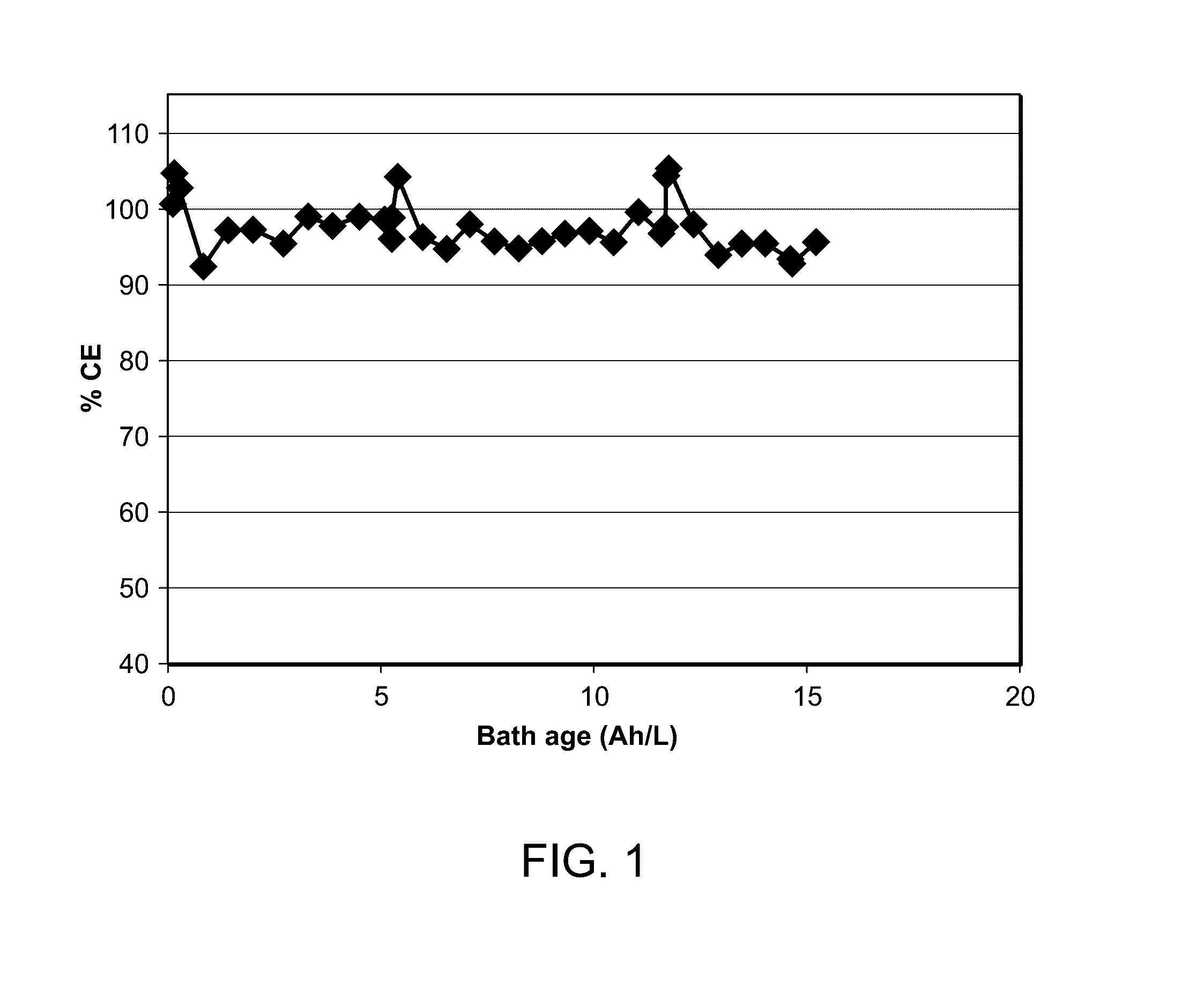
[0048]A plurality of brass panels having dimensions 10×7.5×0.025 cm was degreased and activated as described in Example 1 above. Each panel was then placed in separate Hull cells containing 250 mL of the white bronze bath. The pH of the bath was less than 1. A platinized titanium or bronze electrode was used as anode material. The working bath temperature ranged from 35° C. to 45° C. The panels were electroplated with the white bronze bath at 1 A for 3 minutes. Throughout electroplating all of the baths appeared stable.
[0049]After electroplating the panels were remove...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mole ratio | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| mole ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com