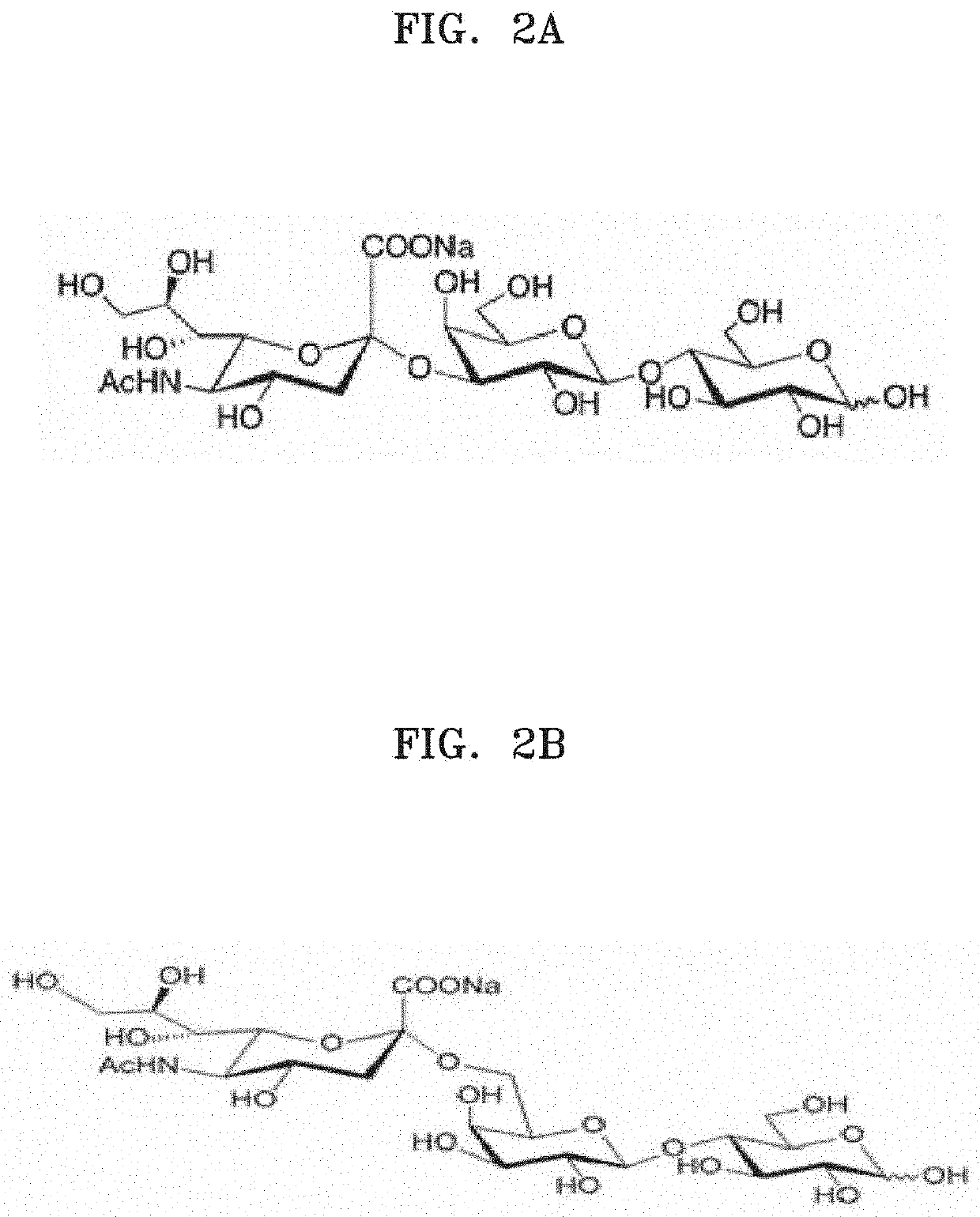
Composition for inhibiting immune cell proliferation comprising sialyllactose or derivative thereof and method thereof
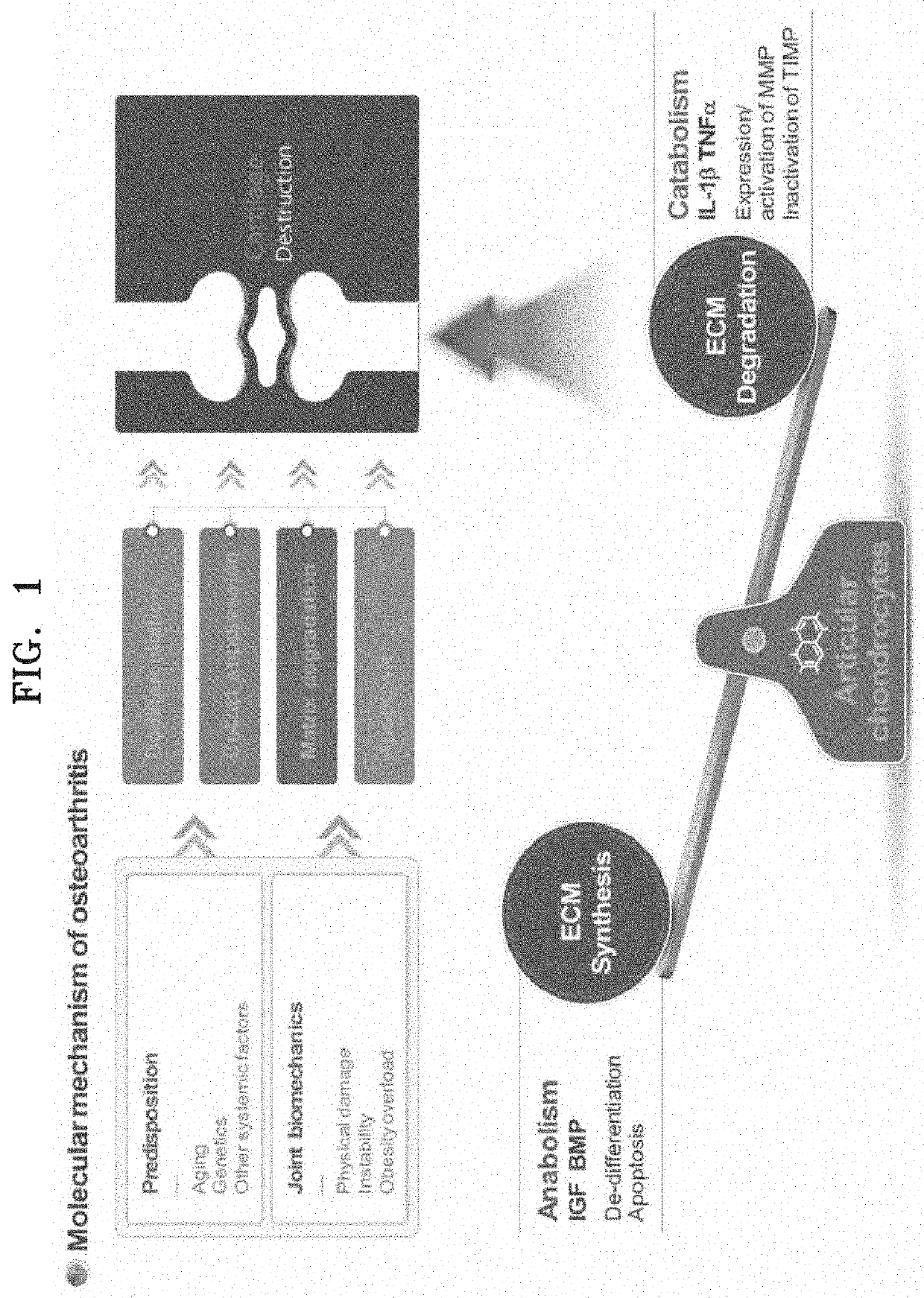
a technology of immune cell proliferation and sialyllactose, which is applied in the direction of dermatological disorders, food ingredients, drug compositions, etc., can solve the problems of affecting the normal regeneration of cartilage tissue that constitutes joints, affecting the normal functioning of cartilage tissue, so as to prevent or treat osteoarthritis, promote cartilage formation, and inhibit the effect of cartilage destruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
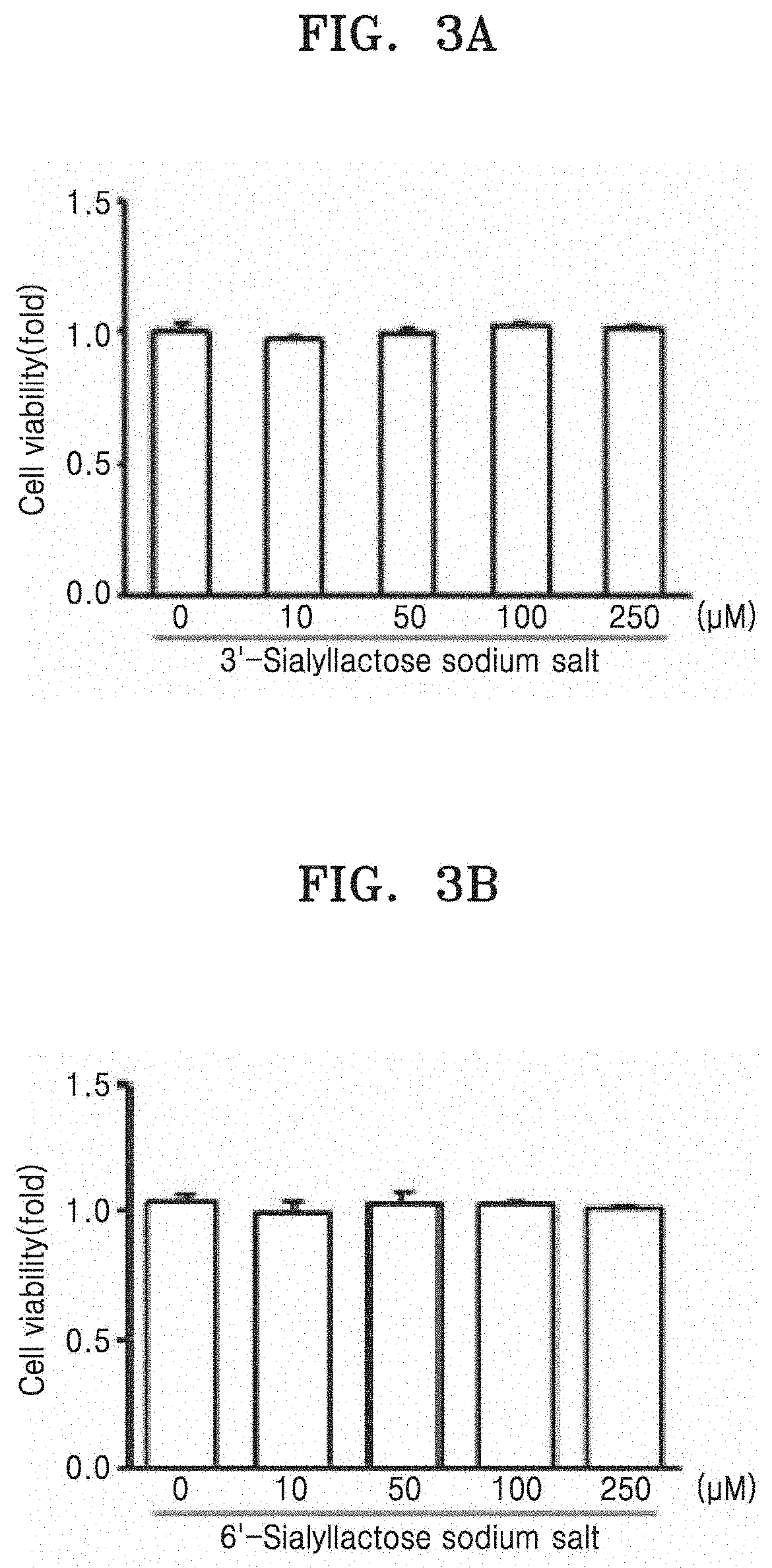
nt of Cytotoxicity of Sialyllactose on Chondrocytes
[0084]Chondrocytes were obtained from cartilage tissues derived from femoral heads, femoral condyles, and tibial plateaus of normal mouse at 5 days after birth. The obtained chondrocytes were cultured in DMEM medium (Gibco, USA) containing 10% (v / v) fetal bovine serum (Gibco, USA), 50 μg / ml of streptomycin (Sigma-Aldrich, USA) and 50 unit / ml of penicillin (Sigma-Aldrich, USA).
[0085]In order to confirm that 3′- or 6′-sialyllactose has no cytotoxicity on chondrocytes, chondrocytes were cultured in a 96-well culture plate at a density of 9×103 cells / well, and then treated with 3′- or 6′-sialyllactose (Genechem Inc., Daejeon, Korea) at a concentration of 0 μM, 10 μM, 50 μM, 100 μM, or 250 μM, followed by incubation in a 5% CO2 incubator at 37° C. for 24 hrs. Cytotoxicity of 3′- or 6′-sialyllactose on chondrocytes was confirmed by measuring absorbance at 450 nm using an EZ-Cytox Cell viability assay kit (DoGen, Korea).
[0086]As a result, ...
example 2
on of Effects of Sialyllactose on Cartilage Formation and Regeneration
2-1: Increase of Expression of Type II Collagen (Col2a1)
[0087]In order to examine effects of 3′- or 6′-sialyllactose on cartilage formation and regeneration, the chondrocytes obtained in Example 1 were incubated for 36 hrs and then treated with 3′- or 6′-sialyllactose at a concentration of 0 μM, 10 μM, 50 μM, 100 μM, or 250 μM, followed by further incubation for 36 hrs.
[0088]Next, in order to perform qRT-PCR, RNA was extracted from the chondrocytes using a TRI reagent (Molecular Research Center Inc.), and cDNA obtained by reverse transcription of RNA was amplified by PCR using primers of SEQ ID NOS: 1 and 2 under condition of annealing temperature of 55° C. to examine expression of type II collagen (Col2a1, 173 bp) which is essential for cartilage formation. As a control group, Gapdh (450 bp, annealing temperature of 58° C.) was examined by using primers of SEQ ID NOS: 3 and 4.
SEQ ID NO: 1: 5′-CACACTGGTAAGTGGGGCAA...
example 3
n of Cartilage Formation and Regeneration Signaling Pathways by Sialyllactose
[0094]Col2a1 expression essential for cartilage formation and regeneration is regulated by a transcription factor Sox-9, and therefore, it was examined whether Sox-9 transcription factor is regulated by 3′-sialyllactose.
[0095]A Sox-9 reporter gene was prepared by inserting 48-bp Sox9 binding site in the first intron of human Col2a1 gene into the upstream of SV40 promoter in pGL3 vector (Zhou G et al., J Biol Chem 1998, 12, 14989-97).
[0096]1 μg of the Sox-9 reporter gene was transfected into chondrocytes using lipofectamine 2000 (Invitrogen) for 3 hrs. The transfected cells were co-treated with 5 ng / ml interleukin 1 beta (IL-1β) and 0 μM, 10 μM, 50 μM, 100 μM, or 250 μM of 3′- or 6′-sialyllactose for 24 hrs, and then chondrocytes were recovered to examine Sox-9 activity by luciferase activity.
[0097]As a result, it was confirmed that Sox-9 activity decreased by IL-1β was restored by 3′- or 6′-sialyllactose (F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com