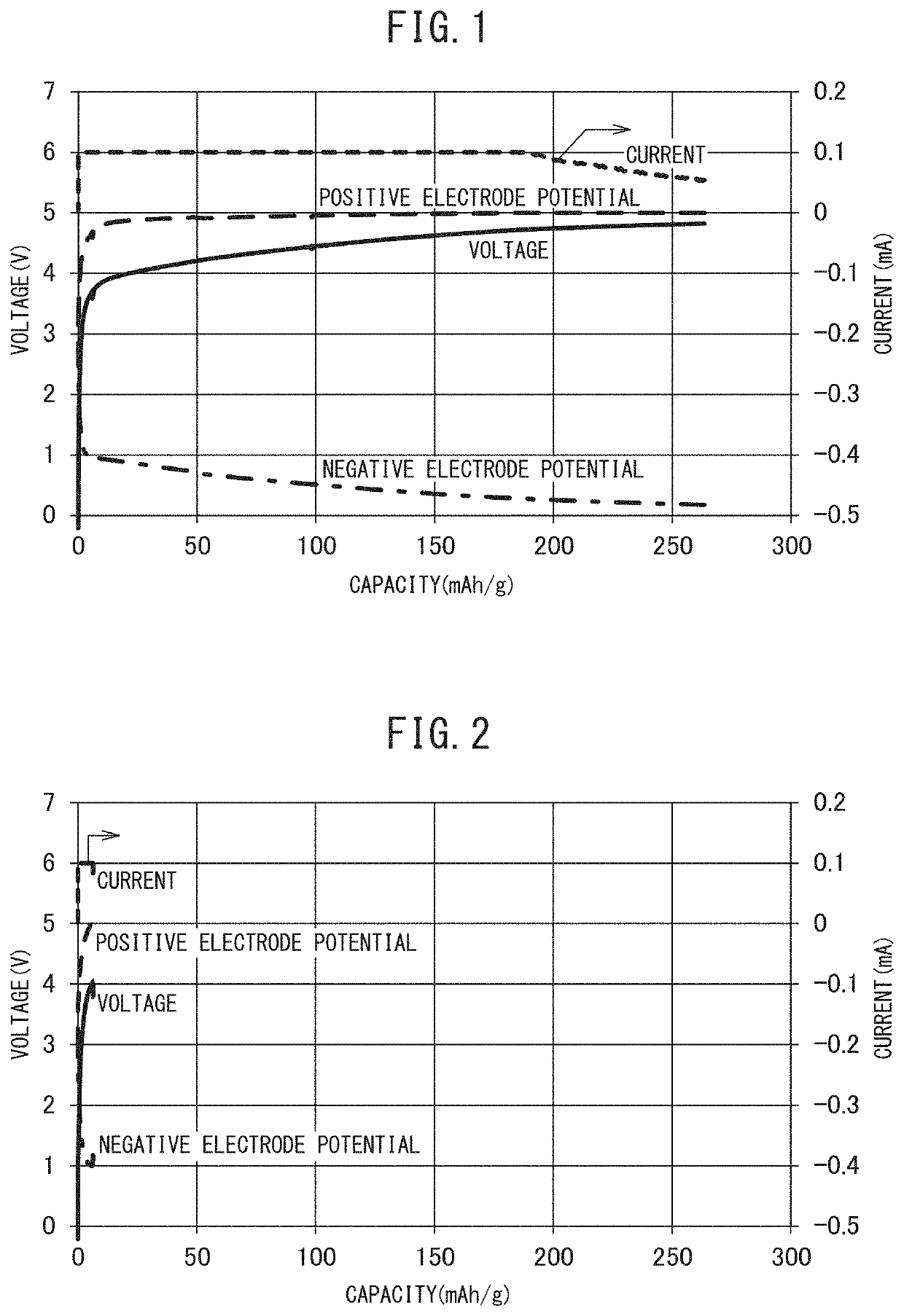
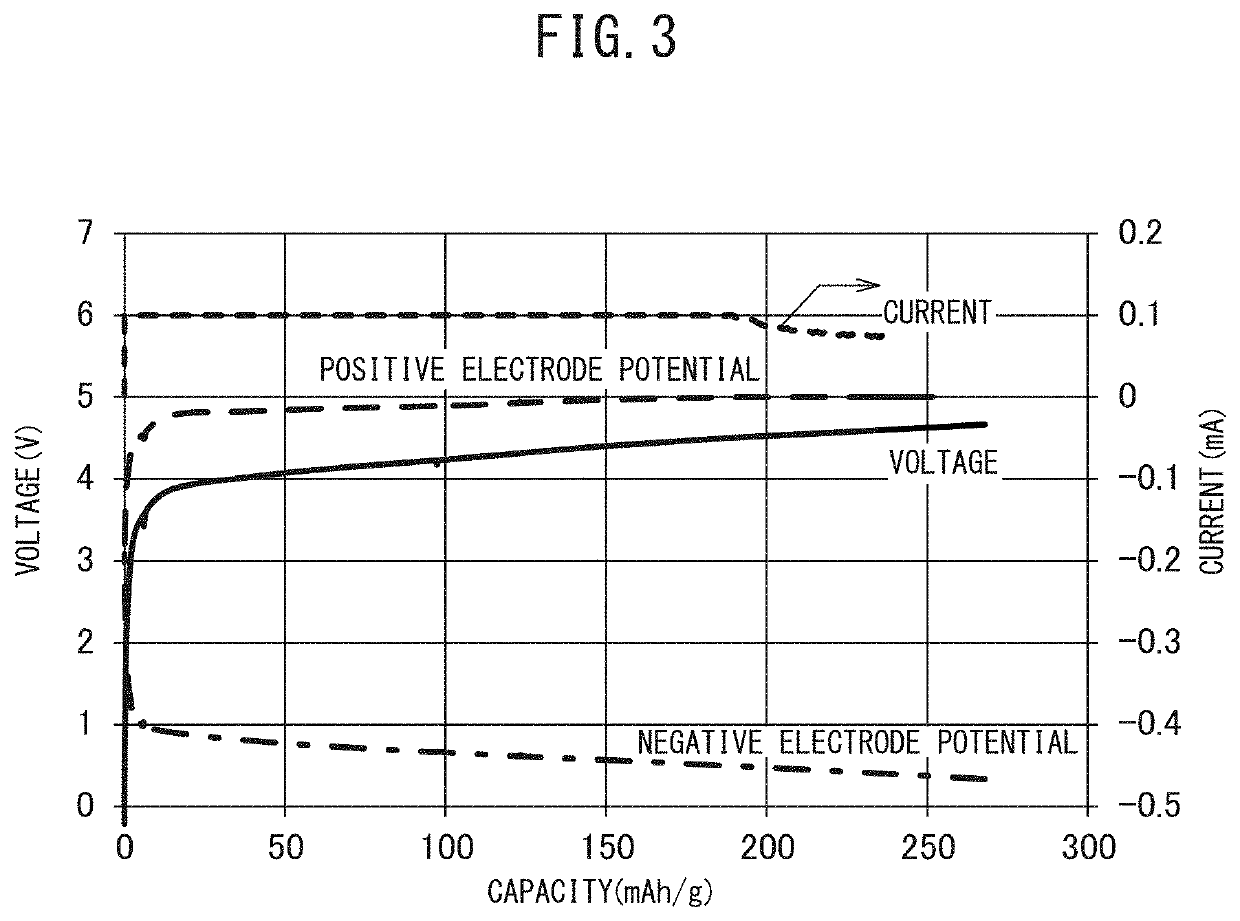
Positive Electrode Precursor
a precursor and electrode technology, applied in the field of positive electrode precursors, can solve the problems of limited use, inferior durability and output characteristics of electric double layer capacitors, and achieve the effects of short time, high capacitance, and promoting decomposition of alkali metal compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Positive Electrode Active Material
preparation example 1a
[0241]A carbide was obtained by carbonization treatment of a crushed coconut shell carbide in a compact-type carbonization furnace at 500° C. for 3 hours under a nitrogen atmosphere. The resulting carbide was put inside an activation furnace where steam which was heated in a preheating furnace was introduced to the activation furnace in a warm state at a rate of 1 kg / h, and activated by increasing a temperature up to 900° C. over a period of 8 hours. The carbide after activation was taken out, and cooled it down under a nitrogen atmosphere, from which an activated carbon was obtained. The resulting activated carbon was washed in a passing water bath for 10 hours. After the activated carbon was dried for 10 hours in an electric drying machine at 115° C., it was crushed for 1 hour using a ball mill, and then activated carbon 1 was obtained.
[0242]The average particle diameter of this activated carbon 1 was 4.2 μm measured by using a laser diffraction-type particle size distribution mea...
preparation example 2a
[0243]A carbide having an average particle diameter of 7 μm was obtained by carrying out carbonization of a phenol resin in a furnace at 600° C. for 2 hours under a nitrogen atmosphere, crushing it using a ball mill, followed by classification of the carbide. Activation was carried out by mixing the carbide and KOH in the weight ratio of 1:5, and heating the mixture at 800° C. for 1 hour in the furnace under a nitrogen atmosphere. Then, activated carbon 2 was obtained by washing it under stirring for 1 hour in diluted hydrochloric acid whose concentration was adjusted to that of 2 mol / L, washing with distilled water under boiling in which pH is held in a range of pH 5 to 6, and then carrying out drying.
[0244]The average particle diameter of activated carbon 2 was 7.0 μm measured by using a laser diffraction-type particle size distribution measurement apparatus (SALD-2000), manufactured by Shimadzu Corp. The fine pore distribution thereof was measured using a fine pore distribution m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com