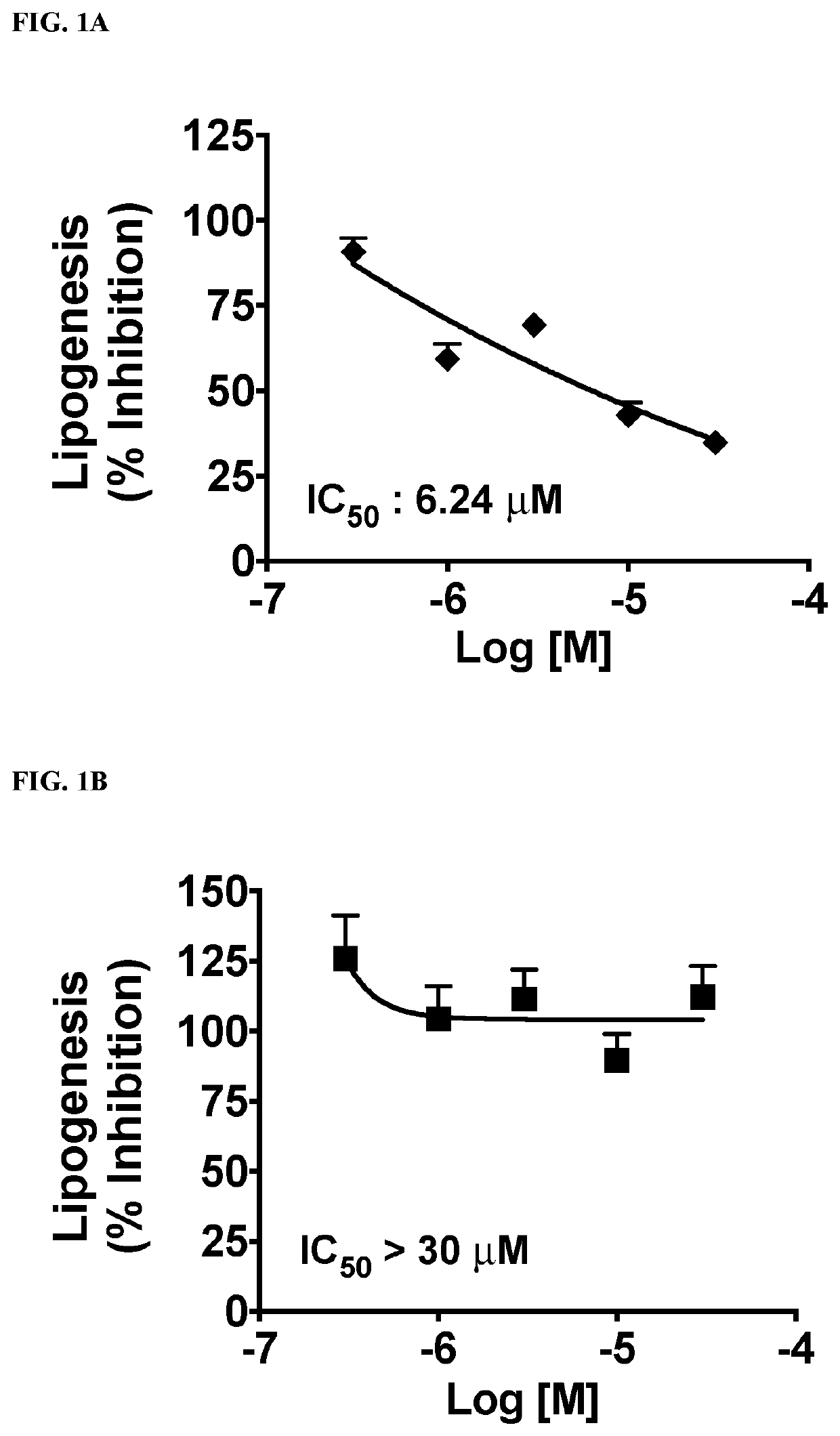
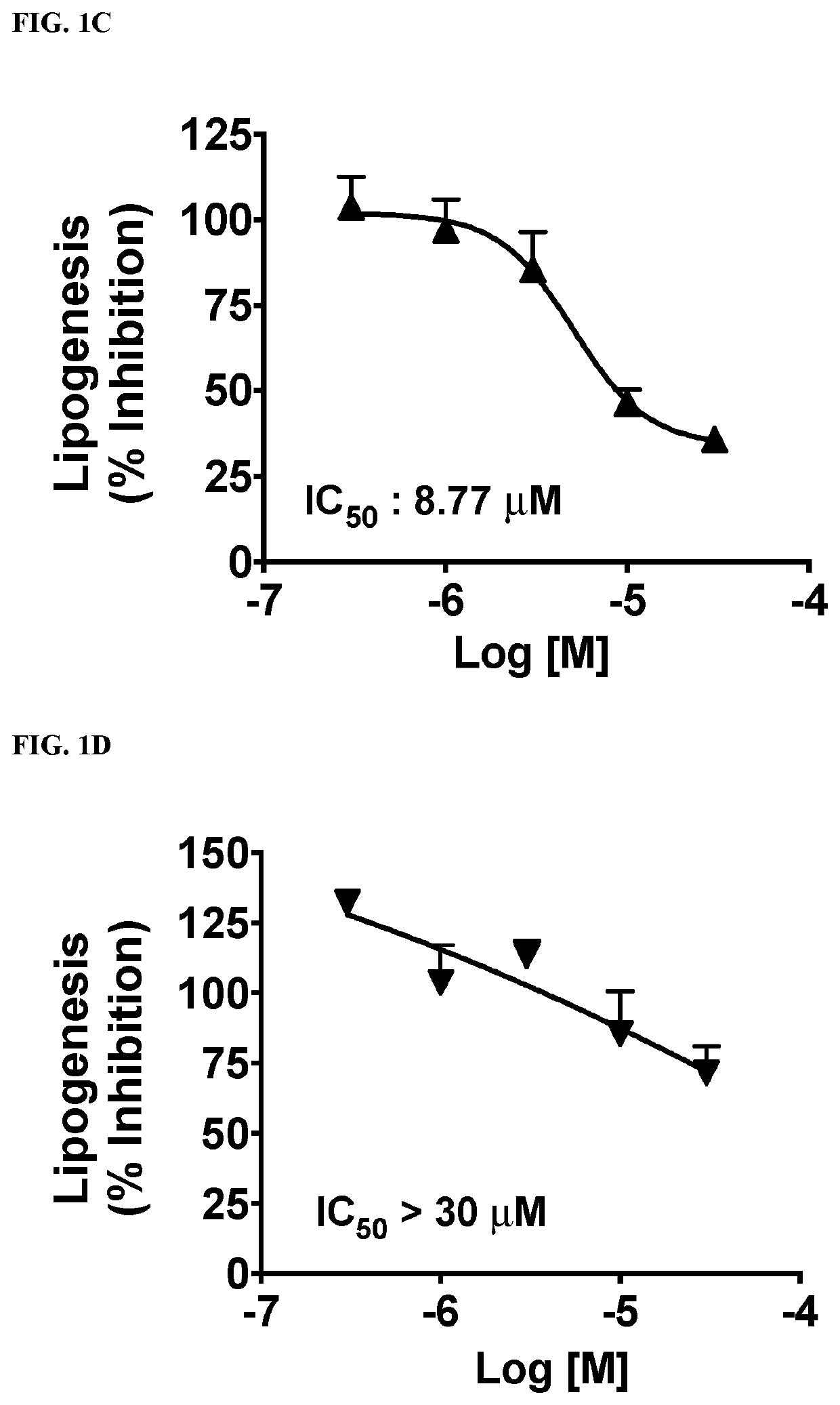
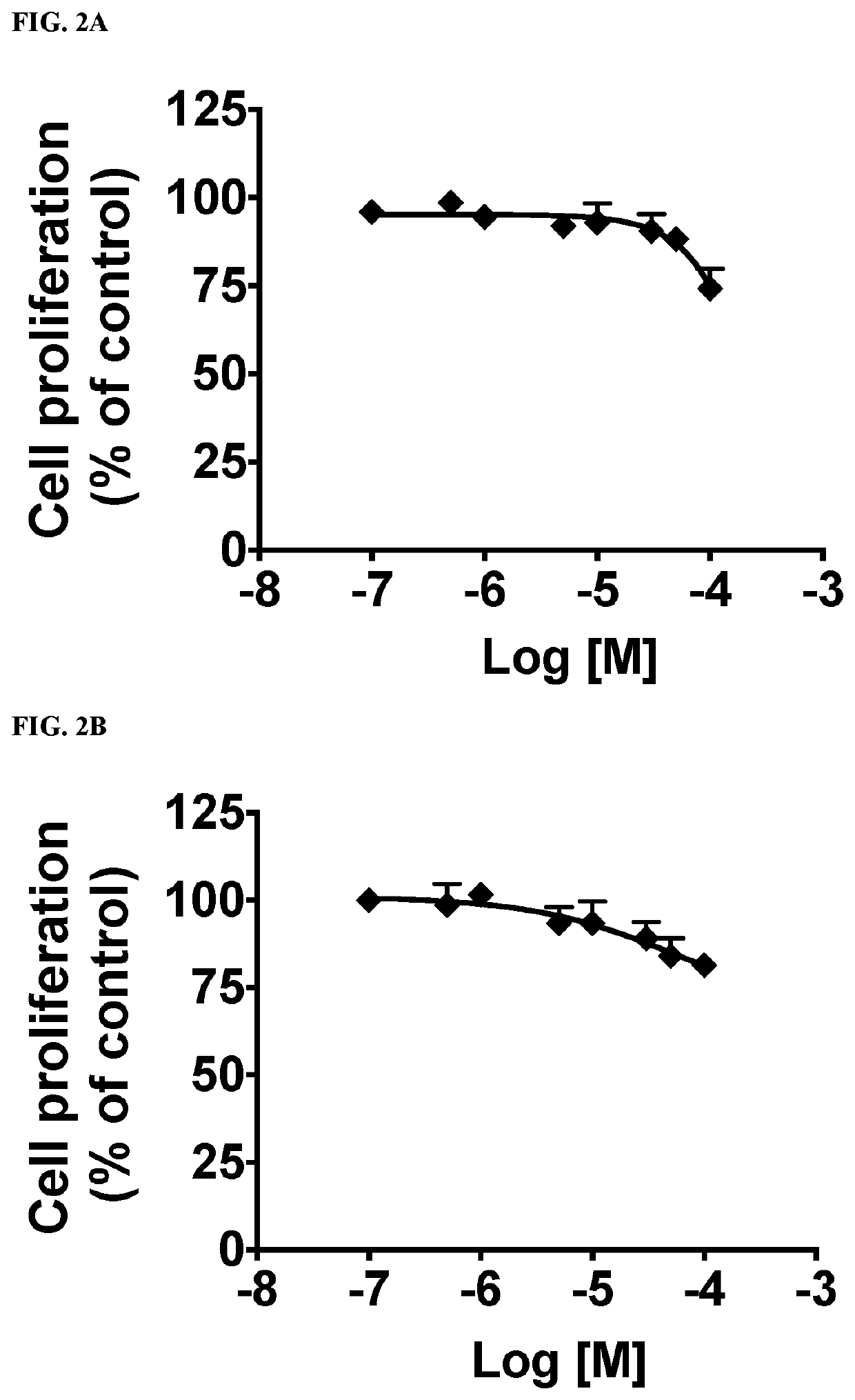
Functionalized long-chain hydrocarbon mono- and di-carboxylic acids and their use for the prevention or treatment of disease
a technology of hydrocarbon mono and dicarboxylic acid and functionalized long chain hydrocarbon, which is applied in the direction of drug composition, organic chemistry, metabolic disorder, etc., can solve the problems of high dose statin therapy that is often not well tolerated, limited treatment options for type iib hyperlipidemia, etc., and achieves the effect of reducing fat or cholesterol conten
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis examples
Example 1
Synthesis of (9-Carboxymethylsulfanyl-5-oxo-nonylsulfanyl)-acetic acid (Compound II-3)
[0526]
[0527]Reaction of ethyl 5-bromovalerate with lithium diisopropylamide in THF at room temperature produces ketone ester 1-1 (see, e.g., Cooke, M. P. J. Org. Chem. 1993, 58, 2910-2912; Stetter, H.; Rauhut, H. Chem. Ber. 1958, 91). Decarboxylation of 1-1 by refluxing in HCl / EtOH (Cooke, M. P. J. Org. Chem. 1993, 58, 2910-2912) produces crude 1-2, which can be purified by column chromatography using systems such as silica gel and mixtures of ethyl acetate / hexanes in ratios from 1 / 20 to 1 / 8. Mercaptoacetic acid dissolved in mixtures of ethanol and water is treated with a solution of sodium hydroxide in water to make sodium mercaptoacetate and is used to treat 1-2 in solvents such as ethanol as described in Agnus, A., Louis, Gissebrecht, J. P., Weiss, R., J. Am. Chem. Soc., 1984, 106, 93 or Riesen, P. C.; Kaden, T. A. Helv. Chim. Acta. 1995, 78, 1325-1333, to provide crude compound II-3. T...
example 2
Synthesis of (9-Carboxymethylsulfanyl-5-hydroxy-nonylsulfanyl)-acetic acid (Compound II-1)
[0528]
[0529]The reduction of ketodiacid compound II-3 from Example 1 is achieved with sodium borohydride after salt formation with NaOH to yield compound II-1 (see U.S. Pat. No. 7,119,221 for suitable reaction conditions). Compound II-3 (Example 1) is dissolved in NaOH solution (2 to 7 equiv) to form an intermediate disodium salt in water. Isopropanol is then added followed by addition of sodium borohydride (1.05 equiv) in portions. The reaction mixture is heated at about 45° C. for a few hours to yield compound II-1. Such product can be purified by recrystallization from MTBE, heptane or mixtures.
example 3
Synthesis of [5-(5-Carboxymethoxy-pentyloxy)-pentyloxy]-acetic acid (Compound II-12)
[0530]
[0531]Compound II-12 is prepared via a Williamson ether synthesis starting from 3-1 and 3-2 (prepared as described in Dasseux et al. U.S. Pat. No. 6,459,003). The resulting 3-3 is deprotected in methanol in the presence of a catalytic amount of p-toluenesulphonic acid monohydrate to give diol 3-4. This diol is then coupled with tert-butyl bromoacetate in a two-phase system of aqueous NaOH and toluene in the presence of tetrabutylammonium bromide as PTC catalyst, as described in U.S. Pat. No. 10,227,285. Finally, this tert-butyl ester is cleaved under acidic conditions to afford compound II-12.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com